Figures & data
Figure 1. General structure of the MPXV genome. The genome is made up of double-stranded linear DNA (approximately 197 kb), primarily composed of hairpin loops, some open reading frames (ORFs), and tandem repeats, while the ITRs are made up of tandem repeats, hairpin loops, and some ORFs [Citation13]. The ends of the genome form direct repeats called ITRs, and the genome has a terminal hairpin loop (no free ends). Most of the essential genes are located in the central part of the genome, and there are ∼250 genes in the genome [Citation14]. The upper box reveals a 625-bp deletion directly upstream of the right ITR (red box), which completely removes MPV-Z-N2R (locus 201) and truncates OMCP (MPV-Z-N3R, locus 202). The central part contains the following genes: D1R: large subunit of mRNA capping enzyme, D2R and D3R: internal structural proteins of intracellular mature virions (IMVs), D4R: viral DNA glycosylase, D5R: ATPase, D6R: subunit of early transcription protein, D7R: subunit of RNA polymerase, D8R: membrane protein of IMV, D9R, D10R, D11R: nucleotide triphosphate phosphorylate, D12R: small subunit of mRNA capping enzyme, and D13R: core protein of IMV [Citation14].
![Figure 1. General structure of the MPXV genome. The genome is made up of double-stranded linear DNA (approximately 197 kb), primarily composed of hairpin loops, some open reading frames (ORFs), and tandem repeats, while the ITRs are made up of tandem repeats, hairpin loops, and some ORFs [Citation13]. The ends of the genome form direct repeats called ITRs, and the genome has a terminal hairpin loop (no free ends). Most of the essential genes are located in the central part of the genome, and there are ∼250 genes in the genome [Citation14]. The upper box reveals a 625-bp deletion directly upstream of the right ITR (red box), which completely removes MPV-Z-N2R (locus 201) and truncates OMCP (MPV-Z-N3R, locus 202). The central part contains the following genes: D1R: large subunit of mRNA capping enzyme, D2R and D3R: internal structural proteins of intracellular mature virions (IMVs), D4R: viral DNA glycosylase, D5R: ATPase, D6R: subunit of early transcription protein, D7R: subunit of RNA polymerase, D8R: membrane protein of IMV, D9R, D10R, D11R: nucleotide triphosphate phosphorylate, D12R: small subunit of mRNA capping enzyme, and D13R: core protein of IMV [Citation14].](/cms/asset/65153be6-7ccb-45d5-a645-be3091ae4f36/temi_a_2132882_f0001_oc.jpg)
Table 1. List of the most important ORFs in the MPXV genome and their functions.
Table 2. List of known MPXV proteins, their encoding genes and host target proteins.
Figure 2. Steps of MPXV entry into host cells [Citation13,Citation14,Citation59–62]. (1) Schematic of the structure of MPXV. (2) Both the EEV and IMV virions penetrate the host membrane by binding and macropinocytosis. MPXV virions use glycosaminoglycans as host receptors. (3) After the internal virion components enter the cytoplasm, core uncoating occurs and this process leads to delivery of the MPXV genome and accessory proteins to the cytosol. (4) The released MPXV genome is used as a template for DNA replication. (5) Early viral DNA transcription followed by translation into the host ribosome occurs to encode essential proteins. Early proteins aid in DNA replication. (6) These proteins interact with host sensor proteins resulting in internal and external modulations. The major intracellular modulations include prevention of viral genome detection, induction of cell cycle arrest, apoptosis inhibition, inhibition of the antiviral system and modulation of some host cellular signalling pathways. Early proteins play essential extracellular roles as immunomodulatory agents and as growth factor-like domains that stimulate onset of mitosis in neighbouring cells. (7) Early proteins are used in production of intermediate proteins. (8) These proteins are involved in late transcription and translation processes and aid in DNA replication. (9) Late proteins are essential components for viral assembly. (10) Viral morphogenesis occurs by formation of inner tubular nucleocapsid structure folding and assembly of viral glycoproteins to generate MV virions. (11) Except those released via infected cell lysis, MV virions transit to the Golgi apparatus along microtubules for double membrane wrapping. (12) The resulting EEV virions exit the infected cell by two routes: by the actin tail assembly, which provides enough force to propel the virions out of the cell or by budding from a cellular membrane (Created with BioRender.com).
![Figure 2. Steps of MPXV entry into host cells [Citation13,Citation14,Citation59–62]. (1) Schematic of the structure of MPXV. (2) Both the EEV and IMV virions penetrate the host membrane by binding and macropinocytosis. MPXV virions use glycosaminoglycans as host receptors. (3) After the internal virion components enter the cytoplasm, core uncoating occurs and this process leads to delivery of the MPXV genome and accessory proteins to the cytosol. (4) The released MPXV genome is used as a template for DNA replication. (5) Early viral DNA transcription followed by translation into the host ribosome occurs to encode essential proteins. Early proteins aid in DNA replication. (6) These proteins interact with host sensor proteins resulting in internal and external modulations. The major intracellular modulations include prevention of viral genome detection, induction of cell cycle arrest, apoptosis inhibition, inhibition of the antiviral system and modulation of some host cellular signalling pathways. Early proteins play essential extracellular roles as immunomodulatory agents and as growth factor-like domains that stimulate onset of mitosis in neighbouring cells. (7) Early proteins are used in production of intermediate proteins. (8) These proteins are involved in late transcription and translation processes and aid in DNA replication. (9) Late proteins are essential components for viral assembly. (10) Viral morphogenesis occurs by formation of inner tubular nucleocapsid structure folding and assembly of viral glycoproteins to generate MV virions. (11) Except those released via infected cell lysis, MV virions transit to the Golgi apparatus along microtubules for double membrane wrapping. (12) The resulting EEV virions exit the infected cell by two routes: by the actin tail assembly, which provides enough force to propel the virions out of the cell or by budding from a cellular membrane (Created with BioRender.com).](/cms/asset/e9b61b55-2d87-4303-9d4f-786772bc0924/temi_a_2132882_f0002_oc.jpg)
Figure 3. MPXV proteins (red) that participate in virostealth, viromimicry and virotransduction are responsible for immune evasion mechanisms of MPX infection [Citation134–137]. In viromimicry, MPXV mimics host receptors that inhibit binding of IFN, IL-1β and TNF as well as MPXV-encoded chemokines and growth factors. In virotransduction, several antiviral pathways including IFN, NF-κB, IRF3 and apoptosis are interfered with by intracellular MPXV-encoded proteins to inhibit their functions. Virostealth is achieved with F1, an anti-apoptotic host range protein that helps with viral replication and the spread of MPX infection (Created with BioRender.com).
![Figure 3. MPXV proteins (red) that participate in virostealth, viromimicry and virotransduction are responsible for immune evasion mechanisms of MPX infection [Citation134–137]. In viromimicry, MPXV mimics host receptors that inhibit binding of IFN, IL-1β and TNF as well as MPXV-encoded chemokines and growth factors. In virotransduction, several antiviral pathways including IFN, NF-κB, IRF3 and apoptosis are interfered with by intracellular MPXV-encoded proteins to inhibit their functions. Virostealth is achieved with F1, an anti-apoptotic host range protein that helps with viral replication and the spread of MPX infection (Created with BioRender.com).](/cms/asset/d3796261-38ee-4e66-b3e0-4b73db87faea/temi_a_2132882_f0003_oc.jpg)
Figure 4. Common symptoms of MPX according to the WHO [Citation160] (Created with BioRender.com).
![Figure 4. Common symptoms of MPX according to the WHO [Citation160] (Created with BioRender.com).](/cms/asset/b56a593f-cdc6-4779-b334-1773652ec555/temi_a_2132882_f0004_oc.jpg)
Figure 5. Transmission routes associated with MPXV according to the WHO [Citation160] (Created with BioRender.com).
![Figure 5. Transmission routes associated with MPXV according to the WHO [Citation160] (Created with BioRender.com).](/cms/asset/0383dfbe-cb41-44e5-9a86-140b56e52f8b/temi_a_2132882_f0005_oc.jpg)
Table 3. Number of MPX cases and deaths from 1970 to 2018.
Table 4. MPX cases and deaths reported by the WHO during the multi-country 2022 outbreak (as of 8 June 2022) [Citation232].
Figure 6. Histology and negative-stain electron microscopic views of MPXV virions (Created with BioRender.com).
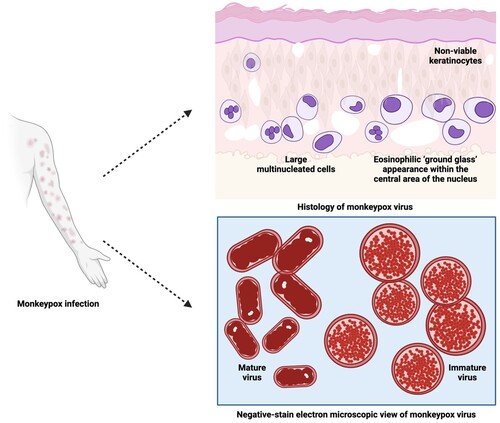
Table 5. List of clinical trials of drugs and vaccines against MPX.
Table 6. Promising anti-MPX medications.
Table 7. Vaccines of various sorts and their potential applications in the prevention and treatment of MPX.
