Figures & data
Figure 1. Construction and characterizations of mpox multi-antigen mRNA vaccine candidates. a, Schematic diagram of formulation procedure for multi-antigen mRNA vaccine candidates. b, Proportions of each antigen mRNA in three batches of Rmix4/6 and Lmix4/6. c-e, Representative images of antigen mRNA expression. Each antigen-encoding mRNA, Rmix4 or Rmix6, was transfected into HEK293T cells. HEK293T cells that expressed M1 were stained with His antibody PE. The other groups of HEK293T cells were stained with each antigen-specific monoclonal antibody and analyzed by flow cytometry. The bindings between antigens and their monoclonal antibody are shown in different colours. Grey shade indicates negative binding.
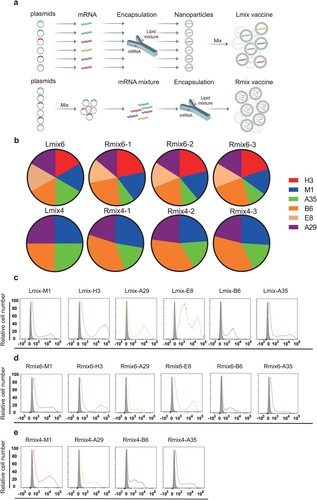
Figure 2. Immunogenicity of multi-antigen mRNA vaccine candidates in mice. Female BALB/c mice (n = 6) were immunized intramuscularly (i.m.) with two doses (1 µg or 5 µg/dose) of multi-antigen mRNA vaccine candidates or with a placebo. a, all six used antigens of mpox (B6, A35, A29, E8, M1, and H3) were mixed at a mass ratio of 1:1:1:1:1:1 and coated in 96-well plates to test antigens-specific antibody titres by ELISA. b, neutralizing activity of sera against MV was determined by plaque reduction neutralization (PRNT) assay against VACV infection, and 50% plaque reduction neutralization (PRNT50) was calculated. c, neutralizing activity of sera against EV was evaluated by comet inhibition assay. d,e, Cellular immune response of female C57BL/6 mice (n = 7) after vaccination with two doses of vaccine (5 µg/dose) or with placebo was measured by ELISPOT assay. IFN-γ and IL-2 secretion of splenocytes after stimulation with VACV were detected to evaluate cellular immune responses. Data are group means ± SEM. P-values were determined with t-test (ns, P > 0.05; *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001).
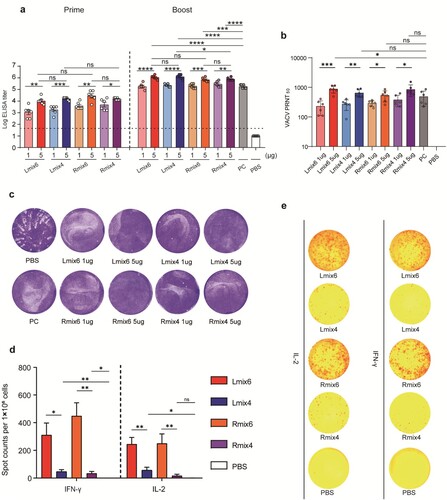
Figure 3. Cross-protective efficacy of mpox multi-antigen mRNA vaccine candidates against fatal VACV challenge in mice. a, Schedule of immunization and viral infection in mice. Female BALB/c mice (n = 6) were immunized i.m. with two doses (1 µg or 5 µg/dose) of multi-antigen mRNA vaccine candidates, 14 days apart. On day 14 following the second immunization, mice were infected intraperitoneally (i.p.) with 5 × 106 PFU of VACV, and changes in body weight (b) and survival (c) of mice were monitored for 14 days.
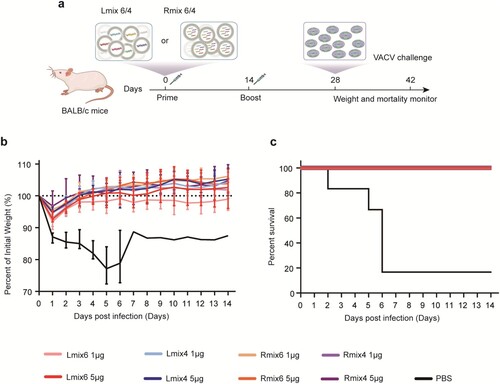
Figure 4. BCR repertoires elicited by mpox individual antigen vaccinations in mice. Female BALB/c mice (n = 5) were immunized i.m. with two doses of each antigen-encoding mRNA vaccine candidate or with a placebo, 14 days apart. Serum samples were collected on day 28. a, neutralizing activity of sera induced by each antigen-encoding mRNA vaccination was detected by plaque reduction neutralization assay against VACV. 90% plaque reduction neutralization (PRNT90) was calculated. b, Summary of the statistics for the scBCR-seq. c, Pie charts show clonal expansion in B6, A35, A29, E8, M1, and H3 specific BGC cells for IGVH (upper panel) or IGVL (bottom panel). Coloured slices are proportional to the number of clonal relatives. d, Antibody characteristics of the top 20 frequent mAbs in antibody repertoire elicited by M1 mRNA vaccine vaccinations. The dark blue, red, light blue, and orange indicate the antibody characteristics of expression, binding, neutralization, and complete competition against reference 7D11, respectively. Antibody expression, binding, and competition characteristics were assayed by Octet. Neutralizing activity of each mAb-expressed cell supernatant was determined by plaque reduction neutralization (PRNT) assay. Data are group means ± SEM. P-values were determined with t-test (ns, P > 0.05; *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001).
Supplemental Material
Download MS Word (3.7 MB)Data availability
The authors declare that the data supporting the findings of this study are available within this paper or are available from the corresponding author upon reasonable request.
