Figures & data
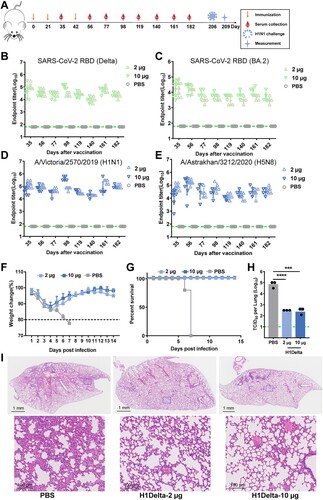
Figure 1. H1Delta protein vaccine design and structure. (A) Schematic of the H1Delta vaccine design. SP, signal peptide. (B) Representative BIAcore diagrams of H1Delta bound to the SARS-CoV-2 RBD antibody CB6 and influenza HA antibody CR9114. The KD value was calculated using BIAevaluation Version 4.1 (GE Healthcare). (C) Cryo-EM structure of the chimeric protein in complex with the RBD-targeting CB6 antibody and the HA-stalk-targeting CR9114 antibody. (D) Overall structure of the trimeric H1Delta.
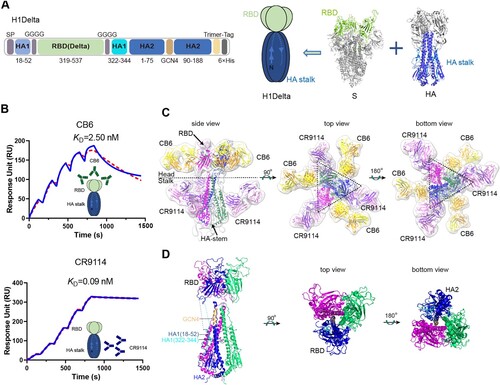
Figure 2. Immunogenicity of the H1Delta vaccine in mice. (A) ELISA assays showing the H1-specific IgG titers. (B) ELISA assays showing the H5-specific IgG titers. (C) ELISA assays showing the SARS-CoV-2 Delta-specific IgG titers. (D-F) 50% neutralization titer of pseudotyped virus (Delta, BA.2, and BA.4/5) in serum. P-values were analyzed with two-tail unpaired t test (*p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001).
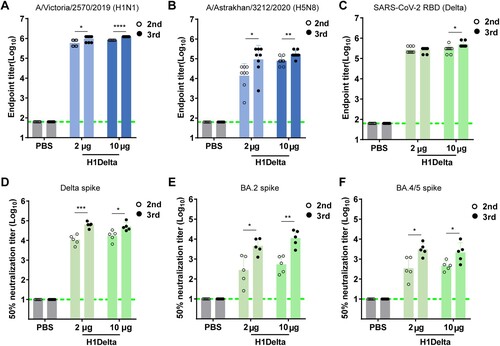
Figure 3. Protection efficacy of the H1Delta protein vaccine to homologous H1N1 and heterologous H5N8. (A-D) Groups of 6-to-8-week-old female BALB/c mice (n = 8) were vaccinated with three immunizations of 2 or 10 μg immunogen with an adjuvant of AddaVax in 3-week intervals. PBS with the adjuvant was given as a control. Serum samples were collected 35 and 56 days after initial immunization. Mice were intranasally challenged with 20 LD50 of A/Brisbane/02/2018 (H1N1) virus. Lung tissues were harvested and split for virus titer detection (n = 3) and pathological examination (n = 3), respectively. Vaccine efficacy was assessed by measuring (A) morbidity (weight loss), (B) mortality (survival), (C) lung viral titers on day 3 post challenge, and (D) histological pathology analyses. (E-H) Groups of 6-to-8-week-old female BALB/c mice (n = 8) were vaccinated with three immunizations of 2 or 10 μg immunogen with an adjuvant of AddaVax in 3-week intervals. PBS with the adjuvant was used as a control. Serum samples were collected 35 and 56 days after initial immunization. Mice were intranasally challenged with 10 LD50 of reassortment A/Astrakhan/3212/2020(H5N8) virus. Lung tissues were harvested and split for virus titer detection (n = 3) and pathological examination (n = 3), respectively. Vaccine efficacy was assessed by measuring (E) morbidity (weight loss), (F) mortality (survival), (G) lung viral titers on day 3 post challenge, and (H) histological pathology analyses. Differences were compared using two-tail unpaired t test (**p < 0.01 and ***p < 0.001).
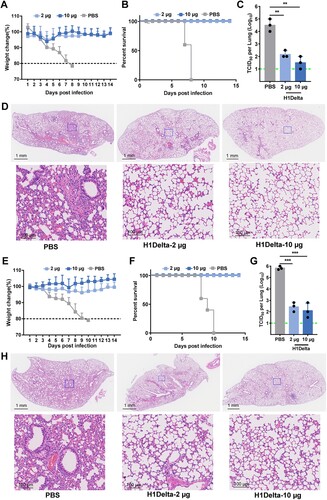
Figure 4. Protection efficacy of the H1Delta protein vaccine to SARS-CoV-2. (A-E) Random selection of five mice in each group that were challenged with 6 × 105 TCID50 of Delta SARS-CoV-2 variant, and (F-G) the other five were challenged with 6 × 105 TCID50 of Omicron (BA.2) variant at 140 days after the primary immunization. Ad5-hACE2 was intravenously administered 5 days before the mice were given the Delta variant challenge. (A) Pulmonary Delta viral gRNA levels were detected by qRT-PCR. (B) Pulmonary Delta viral sgRNA levels were detected by qRT-PCR. (C) Turbinate Delta viral gRNA levels were detected by qRT-PCR. (D) Turbinate Delta viral sgRNA levels were detected by qRT-PCR. (E) Plots showing correlations and corresponding two-sided p values between the pVNT50 of Delta variant (serum samples were collected 140 days after initial immunization) and Delta viral gRNA. (F) Pulmonary Omicron viral gRNA levels were detected by qRT-PCR. (G) Pulmonary Omicron viral sgRNA levels were detected by qRT-PCR. (H) Turbinate Omicron viral gRNA levels were detected by qRT-PCR. (I) Turbinate Omicron viral sgRNA levels were detected by qRT-PCR. (J) Plots showing correlations and corresponding two-sided p values between the pVNT50 of Omicron variant (serum samples were collected 140 days after initial immunization) and Omicron viral gRNA. (K) Histological pathology analyses of lung sections of mice challenged with Delta or Omicron Differences were compared using two-tail unpaired t test (***p < 0.001 and ****p < 0.0001).
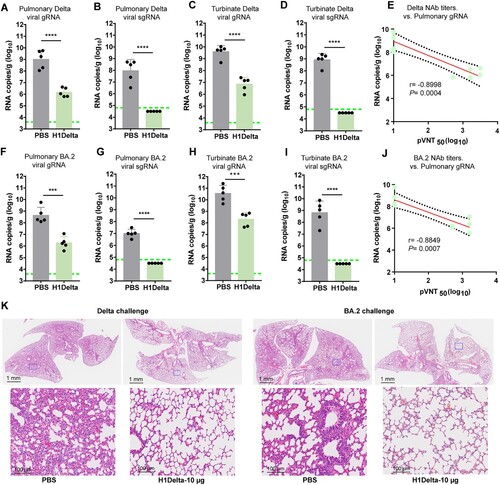
Figure 5. Long-lasting protection of the H1Delta protein vaccine. (A) Time course of H1Delta vaccine immune antibody monitoring, viral challenge, and measurement. (B-E) ELISA assay showing the SARS-CoV-2 Delta (B), BA.2 (C), IAV H1N1 (D), and H5N8 (E) specific IgG titers. (F) Body weight changes at 14 days after virus infection. (G) Mouse survival rates were monitored for 14 days. (H) Virus titers in lungs were detected. (I) Histological pathology analyses of lung sections of mice challenged. Differences were compared using two-tail unpaired t test (***p < 0.001 and ****p < 0.0001).