Figures & data
Figure 1. EV-A71 infection upregulated TRIB3 expression. (a) HCT-8 cells, RD cells and FHC cells were infected with EV-A71 (MOI = 1.0) and harvested at the indicated time post infection. The mRNA level of TRIB3 was analyzed by qRT-PCR (n = 3). P < 0.05, two-way ANOVA with Holm-Sidak multiple comparisons test. (b) EV-A71 infection increased TRIB3 expression in vitro in a time dependent manner. HCT-8 cells, RD cells and FHC cells were infected with EV-A71 (MOI = 1.0) and harvested at the indicated time post infection. Cell extracts were applied for WB assay. (c) EV-A71 infection increased TRIB3 expression in vitro in an inoculum dose dependent manner. HCT-8 cells, RD cells and FHC cells were infected with EV-A71 at the indicated MOI (MOI = 0.01, 0.1, 1.0) and harvested at 10 h post infection. Cell extracts were applied for WB assay.
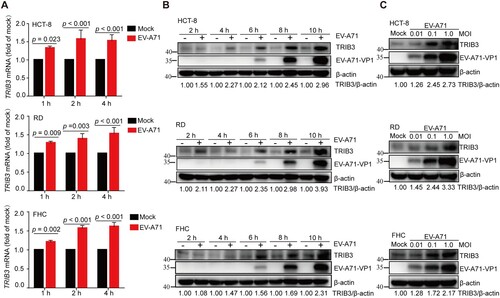
Figure 2. TRIB3 enhances EV-A71 replication. (a to e) TRIB3 overexpression promoted EV-A71 infection. HCT-8 cells were transfected with control-HA or TRIB3-HA plasmids. At 24 h after transfection, HCT-8 cells were mock-infected or infected with EV-A71 (MOI = 0.01) for 24 h. The cells were harvested for WB assay with indicated antibodies (a, n = 3), In-cell WB assay with indicated antibodies (b), IF (c), qRT-PCR assay (d, n = 3) or virus titer assay (e, n = 3) 24 h post infection. (f to j) TRIB3 depletion reduced the replication of EV-A71. Parental or TRIB3-KO HCT-8 cells were infected with EV-A71 (MOI = 0.1) for 24 h. The cells were harvested for WB assay with indicated antibodies (f, n = 3), In-cell WB assay with indicated antibodies (g), IF (h), qRT-PCR assay (i, n = 3) or titer assay (j, n = 3) 24 h post infection. (k) TRIB3-KO cells were transfected with control-HA or TRIB3-HA plasmids. At 24 h after transfection, TRIB3-KO cells were mock-infected or infected with EV-A71 (MOI = 0.1) for 24 h. The cells were harvested for WB assay with indicated antibodies. P < 0.05, two-way ANOVA with Holm-Sidak multiple comparisons test (e, j) or Student’s t-test (a, d, f, i).
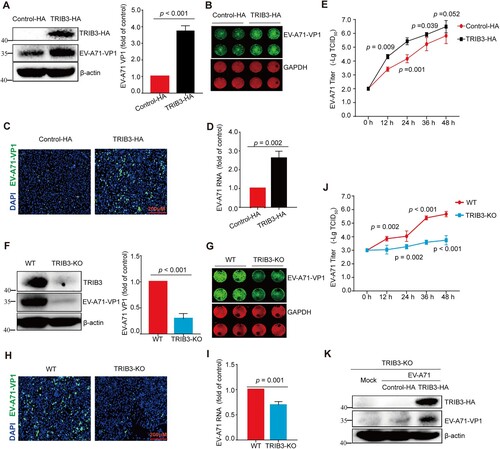
Figure 3. TRIB3 does not interact with viral proteins. (a) 293 T cells were co-transfected with a plasmid expressing Myc-tagged TRIB3 and plasmid expressing HA-tagged VP1, 2A, 2B, 2C, 3A, 3C or GFP-tagged VP2-4, 3B, 3D protein or V5-tagged 2A. Immunoprecipitation from cell lysates were performed and probed with the indicated antibodies. (b) 293 T cells were transfected with plasmid expressing TRIB3-Myc or NEDD8-Myc, the cells were infected with EV-A71 at 24 h post transfection and harvested at 24 h post infection. Immunoprecipitation from cell lysates were performed and probed with the indicated antibodies.
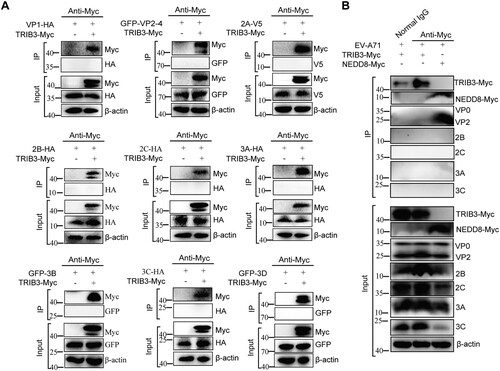
Figure 4. TRIB3 enhances SCARB2 expression and EV-A71 attachment to cells. (a) TRIB3 depletion decreased the protein level of SCARB2. Tandem Mass Tag™-LC-MS/MS analysis evaluated the protein level of SCARB2 in WT cells and TRIB3-KO cells (n = 2). (b, c, g, i) TRIB3 depletion decreased the protein level of SCARB2. SCARB2 expression were detected with WB (b, n = 3), qRT-PCR assay (c, n = 4), Immunofluorescence (g) and flow cytometry (i, n = 3) in WT cells and TRIB3-KO cells. (d, e, f, h) TRIB3 overexpression increased the protein level of SCARB2. HCT-8 cells were treated with Control-HA or TRIB3-HA plasmids. At 24 h after transfection, cells were harvested for WB assay with indicated antibodies (d, n = 3), qRT-PCR assay (e, n = 4), Immunofluorescence (f) and flow cytometry (h, n = 3). Percentage of SCARB2 positive cells was calculated with FCS express software. (j, k) TRIB3 overexpression promoted EV-A71 binding and TRIB3 knockout decreased EV-A71 binding. HCT-8 cells were transfected with indicated plasmids. At 24 h after transfection, HCT-8 cells were rested at 4°C for 1 h and infected with EV-A71 (MOI = 1.0) on ice for 30 min. The cells were rinsed with PBS and harvested. Cell-associated EV-A71 RNA were measured by a qRT-PCR assay (j. n = 3). WT cells and TRIB3-KO cells were rested at 4°C for 1 h and then infected with EV-A71 (MOI = 1.0) on ice for 30 min. The cells were rinsed with PBS and harvested. Cell-associated EV-A71 RNA were measured by were a qRT-PCR assay (k, n = 3). P < 0.05, Student’s t-test (b, c, d, e, h, i, j, k).
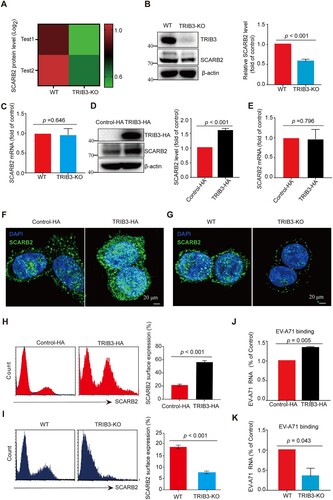
Figure 5. TRIB3 represses ubiquitylation and degradation of SCARB2. (a) 293 T cells were co-transfected with a plasmid expressing SCARB2-Myc and TRIB3-HA or a vector plasmid. At 24 h post transfection, cells were incubated with cycloheximide (CHX) (10 μg/ml) for indicated times. Proteins were detected by WB with the indicated antibodies. P < 0.05, two-way ANOVA with Holm-Sidak multiple comparisons test. (b) The effect of TRIB3 overexpression on SCARB2 ubiquitylation in vitro. 293 T cells were transfected with indicated plasmids and cell extracts were IP with anti-Myc Ab. The ubiquitylated SCARB2 was detected with WB assay. (c, d) 293 T cells were transfected with indicated plasmids and cell extracts were IP with anti-Myc Ab or anti-Flag Ab. The ubiquitylated SCARB2 was detected with WB assay. (e) The KDC region of TRIB3 was responsible for its promoting EV-A71 infection. (Upper panel) Schematic diagram of TRIB3 deletion mutants. (Lower panel) Cell extracts from 293 T cells transfected with the indicated plasmids and infected with EV-A71 were resolved by SDS-PAGE, proteins were detected by WB with the indicated antibodies. (f) Cell extracts from TRIB3-KO cells transfected with the indicated plasmids and infected with EV-A71 were resolved by SDS-PAGE, proteins were detected by WB with the indicated antibodies. (g) Effect of KDC deletion in TRIB3 on SCARB2 ubiquitination in 293 T cells. (h) Cell extracts from 293 T cells transfected with the indicated plasmids and infected with EV-A71 were detected by WB with the indicated antibodies. (i) The relationship between TRIB3 and SCARB2. 293 T cells were transfected with indicated plasmids and cell extracts were IP with anti-HA or anti-Flag Ab. (j) The relationship between endogenous TRIB3 and SCARB2. HCT-8 cells extracts were IP with anti-TRIB3.
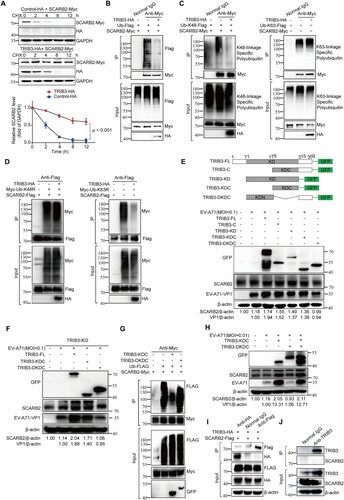
Figure 6. TRIB3 facilitates EV-A71 replication in a SCARB2-independent manner. (a) HCT-8 cells were transfected with control-HA or TRIB3-HA plasmids. At 3 h or 10 h post transfection, HCT-8 cells were harvested for WB assay with indicated antibodies. (b) HCT-8 cells were transfected with control-HA or TRIB3-HA plasmids. At 3 h post transfection, HCT-8 cells were mock-infected or infected with EV-A71 (MOI = 1.0) for 7 h. The cells were harvested for WB assay with indicated antibodies. (c) 293 T cells were transfected with control-HA or TRIB3-HA plasmids. At 24 h post transfection, the cells were transfected again with EV-A71 subgenomic replicon RNA and luciferase reporter activities were determined at different time (n = 6). (d) HCCLM3 cells and SCARB2-KO HCCLM3 cells were harvested for WB assay with indicated antibodies. (e) SCARB2-KO HCCLM3 cells were transfected with control-HA or TRIB3-HA plasmids. At 24 h post transfection, the cells were transfected again with EV-A71 subgenomic replicon RNA and luciferase reporter activities were determined at different time (n = 6). (f–g) SCARB2-KO HCCLM3 cells were transfected with control-HA or TRIB3-HA plasmids. At 24 h after transfection, cells were mock-infected or infected with EV-A71 (MOI = 1) for 24 h. The cells were harvested for WB assay with indicated antibodies (f, n = 3) and qRT-PCR assay (g, n = 3). P < 0.05, two-way ANOVA with Holm-Sidak multiple comparisons test (c, e) or Student’s t-test (f, g).
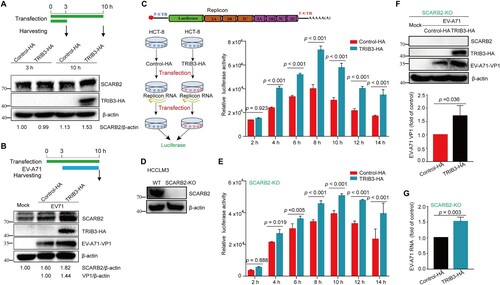
Figure 7. Trib3 knockdown significantly reduce the lethality and severity of EV-A71 infection in mice. (a) Schematic presentation of animal experiment design. (b, c) Trib3 knockdown could delay the death of mice upon lethal EV-A71 challenge and photos were taken at 5 dpi. (d) 12-day C57BL/6 WT mice and Trib3−/+ mice were infected with 1 LD50 of EV-A71, mice survival was observed every day until day 12. (e, f) 12-day C57BL/6 WT mice and Trib3−/+ mice were infected with 0.1 LD50 of EV-A71, mice were weighed daily (e) and observed for clinical scores for 13 days (f). P < 0.05, A Log-Rank (Mantel-Cox) test (c, d) or Ridit assay (f).
Table 1. Impact of Trib3 knockdown on mortality rates and mean survival time (MST) of mice infected with lethal EV-A71.
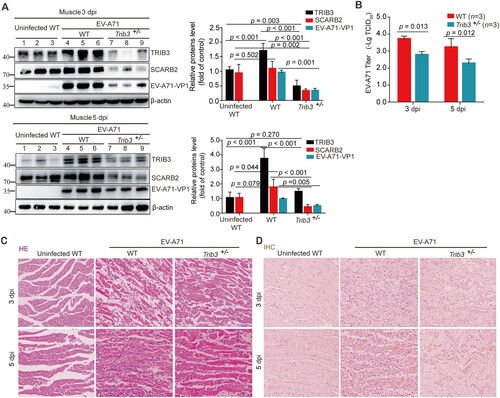
Figure 8. Trib3 knockdown significantly attenuated EV-A71 replication in mice. (a to d) 12-day C57BL/6 WT mice and Trib3−/+ mice were infected with 1 LD50 of EV-A71. Three mice enrolled in each group were dissected at 3 or 5 dpi. Muscle tissues proteins were detected by WB assay with indicated antibodies (a) and viral titer assays (b). Paraffin-embedded sections of muscle tissues were prepared from mice at 3 or 5 dpi and examined with H&E stain (c). The muscle tissue sections prepared from mice at 3 or 5 dpi were stained with EV-A71 VP1 antibody for IHC analyses (d). P < 0.05, two-way ANOVA with Holm-Sidak multiple comparisons test (a, b).