Figures & data
Figure 1. The MGF300-4L-deleted ASFV mutant (Del4L) induces higher IL-1β and TNF-α production than does the wild-type ASFV in primary porcine alveolar macrophages (PAMs). (A and B) Replication characteristics of Del4L in PAMs. PAMs were infected with Del4L, DelCD2v, or the ASFV HLJ/18 strain (ASFV-WT) and the virus yield was titrated at the indicated times and shown as multi-step (MOI = 0.01) (A) or single-step (MOI = 5) (B) growth curve. (C to E) Gene expression profiling in the Del4L-infected PAMs by RNA-seq analysis. PAMs were either mock-infected or infected with Del4L or ASFV-WT (MOI = 5) for 12 and 20 h for RNA-seq analysis. A volcano plot was used to depict the gene expression changes in the Del4L-infected vs the ASFV-WT-infected PAMs (C). Upregulated and downregulated differentially expressed genes (DEGs) were represented by red and blue dots, respectively. Upregulated DEGs were compared between the Del4L and ASFV-WT groups by gene ontology (GO) category functional enrichment analysis. The analysis included three categories: biological process, molecular function, and cell component. Additionally, Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis was performed. The red asterisks indicate the signaling pathways of interest (D). The heatmaps displaying the expression levels of DEGs in the NF-κB signaling pathway induced by Del4L compared with ASFV-WT at 12 and 20 hours postinfection (hpi) (E). (F and G) Del4L induces higher cytokine production than does ASFV-WT in PAMs. PAMs were mock-infected or infected with Del4L or ASFV-WT (MOI = 5). The mRNA levels of IL-1β, TNF-α, and IFN-α (F) in the cell lysates were determined by RT-qPCR at 12 and 20 hpi. The values of the mock-infected group were set to 1 and the values for the infected groups were normalized using those of the mock-infected group. The production of IL-1β, TNF-α, and IFN-α (G) in the cell culture supernatants was detected by commercial ELISA kits at 12 and 20 hpi. * P < 0.05; ** P < 0.01; *** P < 0.001; and ns, not significant (P > 0.05).
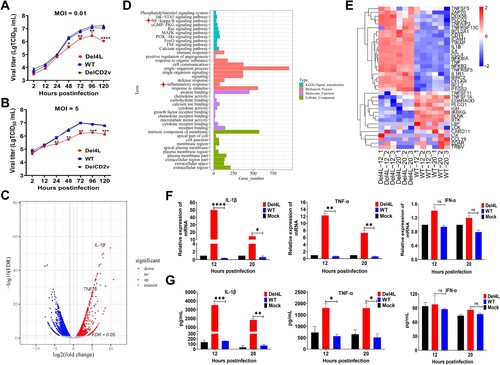
Figure 2. The MGF300-4L protein inhibits the NF-κB signaling pathway. (A to C) The MGF300-4L protein negatively regulates the NF-κB signaling pathway activation. HEK293T cells were cotransfected with the NF-κB reporter plasmids and an increasing amount of pFlag-MGF300-4L (0.1, 0.3, and 0.5 μg). At 24 hours posttransfection (hpt), the cells were either mock-treated or treated with TNF-α at a concentration of 10 ng/mL for 6 h. Subsequently, the luciferase activity was measured. The values of the mock-treated group were set to 1 and the values for the TNF-α-treated groups were normalized using those of the mock-infected group (A). HEK293T cells were transfected with the empty vector (pFlag-Vector) or pFlag-MGF300-4L. The cells were mock-treated or treated with TNF-α at a concentration of 10 ng/mL for 1 h at 24 hpt. The mRNA expression levels of IL-1β and TNF-α were measured by RT-qPCR (B and C). (D and E) The MGF300-4L protein inhibits the nuclear translocation of p65. HEK293T cells were transfected with pFlag-MGF300-4L or pFlag-Vector and then treated with or without TNF-α (10 ng/mL) for 1 h at 24 hpt. The cells were fractionated into cytoplasmic and nuclear fractions and analyzed by immunoblotting (IB) with the indicated antibodies. The p65 in the nuclear and cytoplasmic compartments and the whole cell lysates (WCL) was analyzed by IB. Lamin B1 and β-tubulin were selected as markers for the nuclear and cytosolic fractions, respectively (D). PAMs were either mock-infected or infected with ASFV-WT or Del4L at an MOI of 5. At 20 hours postinfection (hpi), the mock-infected and the ASFV-infected PAMs were treated with or without TNF-α (10 ng/mL) for 1 h. Subsequently, cellular fractions of the ASFV-infected PAMs were separated following the previously described method [Citation26] (E). (F and G) The MGF300-4L protein inhibits the phosphorylation levels of IκBα and p65. HEK293T cells were transfected with pFlag-MGF300-4L or pFlag-Vector and then treated with or without TNF-α (10 ng/mL) for 30 min at 20 hpt. The phosphorylation levels of IκBα and p65 were analyzed by IB with the indicated antibodies (F). The phosphorylation levels of IκBα and p65 in the PAMs mock-infected or infected with ASFV-WT or Del4L (MOI = 5) were analyzed by IB at 12 and 20 hpi (G). * P < 0.05; ** P < 0.01; *** P < 0.001; and ns, not significant (P > 0.05).
![Figure 2. The MGF300-4L protein inhibits the NF-κB signaling pathway. (A to C) The MGF300-4L protein negatively regulates the NF-κB signaling pathway activation. HEK293T cells were cotransfected with the NF-κB reporter plasmids and an increasing amount of pFlag-MGF300-4L (0.1, 0.3, and 0.5 μg). At 24 hours posttransfection (hpt), the cells were either mock-treated or treated with TNF-α at a concentration of 10 ng/mL for 6 h. Subsequently, the luciferase activity was measured. The values of the mock-treated group were set to 1 and the values for the TNF-α-treated groups were normalized using those of the mock-infected group (A). HEK293T cells were transfected with the empty vector (pFlag-Vector) or pFlag-MGF300-4L. The cells were mock-treated or treated with TNF-α at a concentration of 10 ng/mL for 1 h at 24 hpt. The mRNA expression levels of IL-1β and TNF-α were measured by RT-qPCR (B and C). (D and E) The MGF300-4L protein inhibits the nuclear translocation of p65. HEK293T cells were transfected with pFlag-MGF300-4L or pFlag-Vector and then treated with or without TNF-α (10 ng/mL) for 1 h at 24 hpt. The cells were fractionated into cytoplasmic and nuclear fractions and analyzed by immunoblotting (IB) with the indicated antibodies. The p65 in the nuclear and cytoplasmic compartments and the whole cell lysates (WCL) was analyzed by IB. Lamin B1 and β-tubulin were selected as markers for the nuclear and cytosolic fractions, respectively (D). PAMs were either mock-infected or infected with ASFV-WT or Del4L at an MOI of 5. At 20 hours postinfection (hpi), the mock-infected and the ASFV-infected PAMs were treated with or without TNF-α (10 ng/mL) for 1 h. Subsequently, cellular fractions of the ASFV-infected PAMs were separated following the previously described method [Citation26] (E). (F and G) The MGF300-4L protein inhibits the phosphorylation levels of IκBα and p65. HEK293T cells were transfected with pFlag-MGF300-4L or pFlag-Vector and then treated with or without TNF-α (10 ng/mL) for 30 min at 20 hpt. The phosphorylation levels of IκBα and p65 were analyzed by IB with the indicated antibodies (F). The phosphorylation levels of IκBα and p65 in the PAMs mock-infected or infected with ASFV-WT or Del4L (MOI = 5) were analyzed by IB at 12 and 20 hpi (G). * P < 0.05; ** P < 0.01; *** P < 0.001; and ns, not significant (P > 0.05).](/cms/asset/c8e2322e-a185-4564-84bd-e3b1fe884639/temi_a_2333381_f0002_ob.jpg)
Figure 3. The MGF300-4L protein interacts with both IKKβ and IκBα. (A to C) The MGF300-4L protein interacts with the regulatory proteins of NF-κB. HEK293T cells were transfected with pHA-MGF300-4L along with pFlag-IKKα, pFlag-IKKβ, pFlag-NEMO, or pFlag-IκBα as indicated. The cells were lysed and the whole cell lysates (WCL) were immunoprecipitated with an anti-HA monoclonal antibody (mAb) at 36 hours posttransfection (hpt). The immunoprecipitates were examined by immunoblotting (IB) with the indicated antibodies (A). HEK293T cells were transfected with pFlag-IKKβ or pFlag-IκBα. At 36 hpt, the cells were lysed with RIPA buffer. The purified GST or GST-MGF300-4L proteins were used to pull down the IKKβ or IκBα in the lysates and analyzed by IB with the indicated antibodies (B). HEK293T cells were transfected with either pFlag-Vector or pFlag-MGF300-4L. At 20 hpt, the cells were infected with the cell-adapted ASFV for 20 h. Subsequently, the cells were lysed and immunoprecipitation was performed using an anti-Flag or IgG antibody. The WCL and the immunoprecipitates complexes were analyzed by IB (C). (D and E) The ASFV MGF300-4L is colocalized with IKKβ and IκBα. HEK293T cells were transfected with pFlag-MGF300-4L alone or cotransfected with pHA-IKKβ or pHA-IκBα in combination with pFlag-MGF300-4L. IKKβ, IκBα, and MGF300-4L were detected by laser confocal microscopy. The colocalization of MGF300-4L and IKKβ or IκBα was analyzed by the Coloc2 tool of ImageJ/FIJI and shown as Pearson’s correlation coefficients. Scale bar, 20 μm.
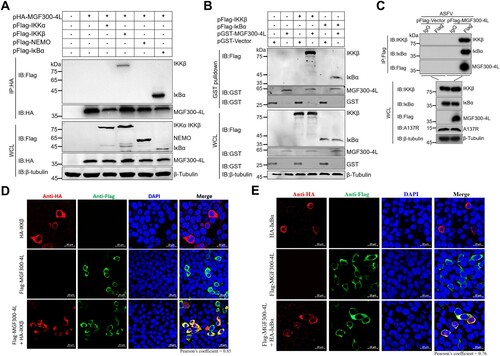
Figure 4. The MGF300-4L protein promotes the autophagic degradation of IKKβ. (A to B) The effects of MGF300-4L on the expression of IKKβ. HEK293T cells were cotransfected with pHA-IKKβ and an increasing amount of pFlag-MGF300-4L (0.5, 1, and 1.5 μg). At 24 hours posttransfection (hpt), the cell lysates were analyzed by immunoblotting (IB) (A). HEK293T cells were transfected with an increasing amount of pFlag-MGF300-4L (0.5, 1, and 1.5 μg). At 24 hpt, the cells were treated with TNF-α (10 ng/mL) for 30 min. The expression level of endogenous IKKβ in the cells was analyzed by IB (B). (C and D) Cycloheximide (CHX) chase assay. HEK293T cells were transfected with pFlag-IKKβ or cotransfected with pFlag-IKKβ and pFlag-MGF300-4L, and then the cells were treated with CHX (100 μg/mL) and harvested at the indicated time points. The cell lysates were analyzed by IB (C). HEK293T cells were transfected with pFlag-Vector or pFlag-MGF300-4L, and then the cells were treated with CHX (100 μg/mL) and harvested at the indicated time points. The expression level of endogenous IKKβ in the cells was analyzed by IB (D). PAMs were either mock-infected or infected with Del4L or ASFV-WT (MOI = 5). At 12 and 24 hours postinfection (hpi), the mRNA level of IKKβ in the cell lysates was determined by RT-qPCR. The values of the mock-infected group were set to 1 and the values for the infected groups were normalized using those of the mock-infected group (E). (F) The effects of MGF300-4L on the expression of IKKβ during ASFV infection. PAMs were mock-infected or infected with ASFV-WT or Del4L (MOI = 5) and analyzed for IKKβ and ASFV-A137R expression levels at 12 and 16 hpi by IB. (G) The effects of inhibitors on the ubiquitin-proteasome and autophagy-lysosome pathways. HEK293T cells were cotransfected with pFlag-IKKβ and pFlag-MGF300-4L. At 24 hpt, the cells were treatment with Lac (20 μM), MG132 (20 μM), BafA1 (100 nM), or 3-MA (10 mM) for 6 h. The cell lysates were analyzed by IB. (H and I) CHX chase assay. HEK293T cells were cotransfected with pFlag-IKKβ and pFlag-MGF300-4L (H) or transfected with pFlag-MGF300-4L (I). Afterwards, the cells were treated with BafA1 (100 nM) and CHX (100 μg/mL), and the cell lysates were analyzed by IB at the indicated time points. * P < 0.05; ** P < 0.01; *** P < 0.001; and ns, not significant (P > 0.05).
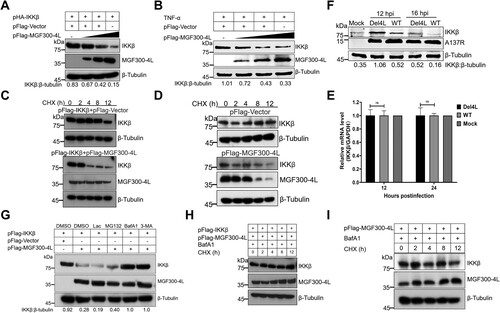
Figure 5. MGF300-4L degrades IKKβ via the chaperon-mediated autophagy. (A to C) MGF300-4L degrades IKKβ independently of macroautophagy. pFlag-IKKβ and pFlag-MGF300-4L were cotransfected into the autophagy-related protein 3-knockout (ATG3-/-) (A), ATG5-/- (B), ATG7-/- (C), or wild-type (WT) HEK293T cells. At 36 hours posttransfection (hpt), the expression levels of IKKβ and MGF300-4L were analyzed by immunoblotting (IB). (D) MGF300-4L interacts with HSC70. HEK293T cells were cotransfected with pFlag-MGF300-4L along with pV5-HSC70. The cells were lysed and the whole cell lysates (WCL) were immunoprecipitated with an anti-Flag monoclonal antibody. The immunoprecipitates were examined by IB with the indicated antibodies. (E and F) MGF300-4L or IKKβ interacts with HSC70 or LAMP2A. HEK293T cells were transfected with pFlag-MGF300-4L or pFlag-IKKβ. At 24 hpt, the cells were lysed and immunoprecipitated by anti-Flag or IgG antibodies. The WCL and the immunoprecipitates complexes were analyzed by IB with the indicated antibodies. (G to I) MGF300-4L degrades IKKβ via CMA. HEK293T cells were transfected with pFlag-MGF300-4L and scramble or HSC70- (G) or LAMP2A-specific siRNAs (H). At 24 hpt, the protein levels of MGF300-4L, endogenous IKKβ, HSC70, and LAMP2A were checked by IB. PAMs were transfected with scramble or LAMP2A-specific siRNAs for 20 h. The protein levels of A137R, endogenous IKKβ, and endogenous LAMP2A were checked by IB (I).
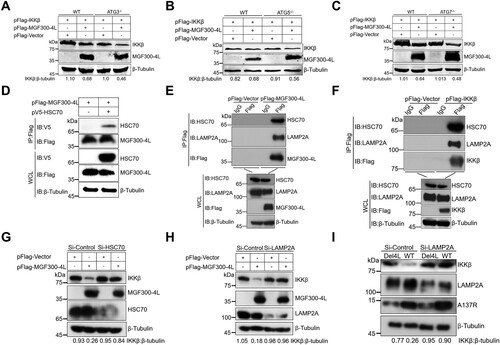
Figure 6. The MGF300-4L protein competes with IκBα for binding to β-TrCP and thereby blocks IκBα degradation. (A to D) The effects of MGF300-4L on the expression of IκBα. HEK293T cells were cotransfected with pHA-IκBα and an increasing amount of pFlag-MGF300-4L (0.5, 1, and 1.5 μg). At 24 hours posttransfection (hpt), the cell lysates were analyzed for the expression of IκBα by immunoblotting (IB) (A). HEK293T cells were transfected with an increasing amount of pFlag-MGF300-4L (0.5, 1, and 1.5 μg). At 24 hpt, the cells were treated with TNF-α (10 ng/mL) for 30 min. The cell lysates were analyzed for the expression of endogenous IκBα by IB (B). HEK293T cells were transfected with pFlag-MGF300-4L or the empty vector (pFlag-Vector). At 20 hpt, the cells were treated with 10 ng/mL TNF-α for the indicated times. The cells lysates were analyzed for IκBα expression by IB (C). HEK293T cells were cotransfected with pHA-IκBα, pMyc-β-TrCP, and an increasing amount of pFlag-MGF300-4L (0.5, 1, and 1.5 μg). At 24 hpt, the cell lysates were analyzed for the ubiquitination degradation of IκBα by IB (D). (E) MGF300-4L interacts with β-TrCP. HEK293T cells were cotransfected with pHA-MGF300-4L along with pMyc-β-TrCP. The cells were lysed and the whole cell lysates (WCL) were analyzed by immunoprecipitation (IP) assay with an anti-HA monoclonal antibody (mAb) at 36 hpt. The immunoprecipitates were examined by IB with the indicated antibodies. (F) MGF300-4L inhibits the ubiquitination of IκBα. HEK293T cells were transfected with pFlag-MGF300-4L and then treated with or without TNF-α (10 ng/mL) for 30 min. At 24 hpt, the cells were examined for IP with an anti-IκBα antibody. The WCL and precipitated proteins were analyzed by IB with the indicated antibodies. (G) The MGF300-4L protein competes with IκBα for binding to β-TrCP. HEK293T cells were cotransfected with pHA-IκBα, pMyc-β-TrCP, and an increasing amount of pFlag-MGF300-4L (0.5, 1, and 1.5 μg). At 24 hpt, the cell lysates were used for IP with an anti-HA antibody, followed by IB with the indicated antibodies. (H) IκBα does not compete with MGF300-4L for binding to β-TrCP. HEK293T cells were cotransfected with pHA-IκBα, pMyc-β-TrCP, and an increasing amount of pFlag-MGF300-4L (0.5, 1, and 1.5 μg). At 24 hpt, the cell lysates were used for IP with an anti-Myc antibody, followed by IB with the indicated antibodies. (I) MGF300-4L inhibits the degradation of IκBα during viral infection. The IκBα level in the PAMs mock-infected or infected with ASFV-WT or Del4L (MOI = 5) followed by treatment with or without TNF-α (10 ng/mL) were analyzed by IB at the indicated time points.
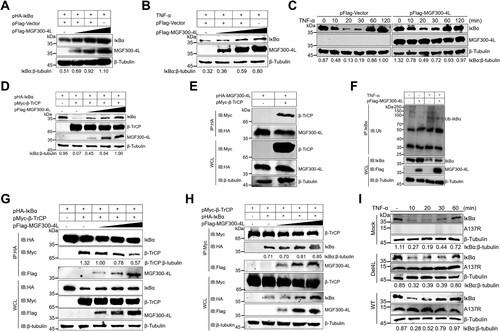
Figure 7. The MGF300-4L protein is associated with the virulence of ASFV. (A to D) Effects of MGF300-4L on ASFV pathogenicity in pigs. The specific-pathogen-free pigs were inoculated intramuscularly (i.m.) with 102.0 TCID50 of Del4L (n = 4) or ASFV-WT (n = 3) or mock-inoculated i.m. with RPMI 1640 (n = 3). The rectal temperatures (A) and survival rates (B) of different groups were recorded. (C and D) Viral loads in the inoculated pigs. Genomic copies in the whole blood (C) and different tissues (D) of the pigs inoculated with ASFV-WT or Del4L were detected by qPCR. LN1: submandibular lymph nodes, LN2: mesenteric lymph nodes. * P < 0.05; ** P < 0.01; *** P < 0.001; and ns, not significant (P > 0.05).
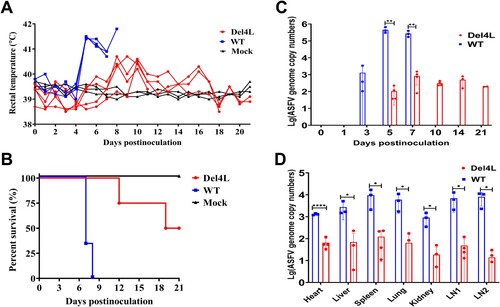
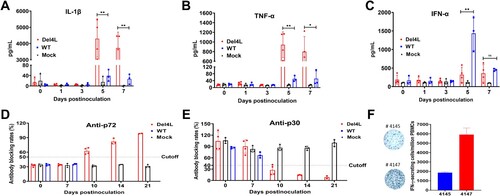
Figure 8. The MGF300-4L gene deletion results in pronounced innate and adaptive immune responses to ASFV infection in pigs. (A to C) Del4L infection induces a strong IL-1β and TNF-α production in pigs. The expression levels of IL-1β (A), TNF-α (B), and IFN-α (C) in the serum samples were measured by commercial ELISA kits. (D and E) Del4L infection induces robust anti-ASFV antibodies in pigs. Serum antibodies against p72 (D) and p30 (E) in the inoculated pigs were detected by ELISA. The results were shown as blocking percentages. The serum sample was regarded as positive when the blocking rate for anti-p72 antibodies exceeded 50% and as negative when the blocking rate below 40%. The sample was regarded as positive for anti-p30 antibodies when the blocking rate was below 40% and as negative when the blocking rate exceeded 50%. The samples exhibiting a blocking rate between 40 and 50% were regarded as doubtful. (F) Del4L infection induces a robust cellular immune response in pigs. The numbers of IFN-γ-producing cells in the peripheral blood mononuclear cells (PBMCs) were measured in the two surviving pigs by enzyme-linked immunospot assay (ELIspot). * P < 0.05; ** P < 0.01; *** P < 0.001; and ns, not significant (P > 0.05).
Supplemental Material
Download Zip (33.8 MB)Supporting_information_updated_V2_1
Download MS Word (21.7 KB)Supplementary_Table1
Download MS Excel (9.4 KB)Data availability statement
The RNA sequence reads generated in this study have been deposited in the sequence read archive database under the accession number PRJNA970972 (BioProject). The complete genome sequence of the ASFV HLJ/18 strain used in this study can be accessed under the GenBank accession no. MK333180.1.
