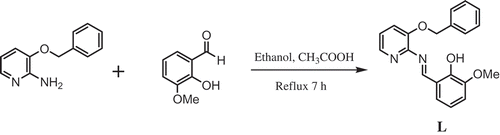
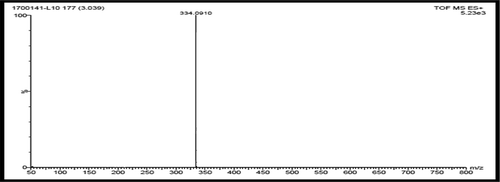
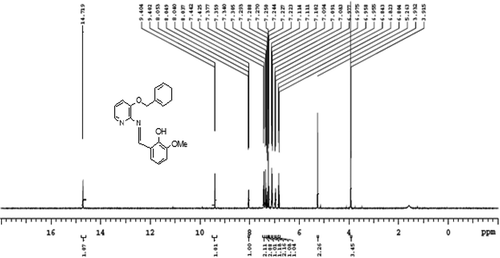
Figures & data
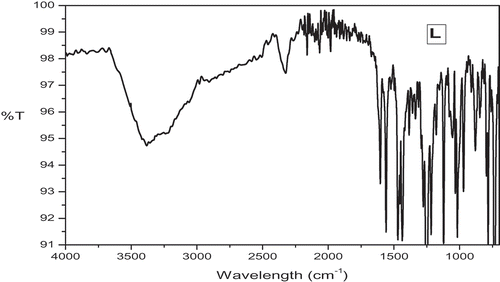
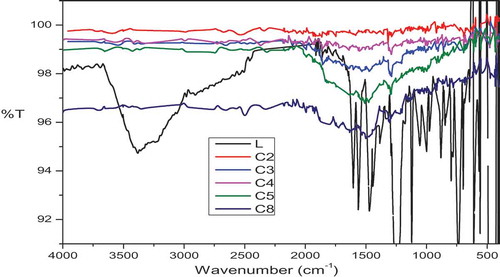
Table 1. FTIR spectral data of the Schiff base ligand [L] and its metal complexes
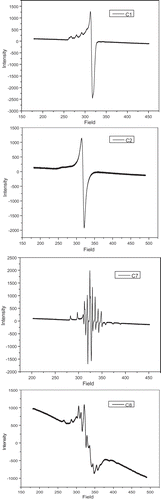
Table 2. EPR spectral parameters for copper and oxidovanadium complexes in DMSO at 77 K
Table 3. Antimicrobial results of the Schiff base ligand and its metal complexes
Table 4. MIC [μg/ml] values for antimicrobial activity of Schiff base ligand and its corresponding metal complexes
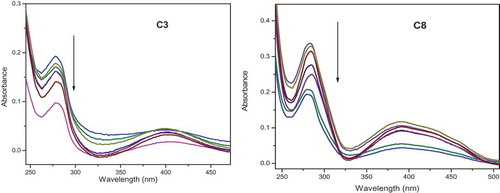
Figure 11. Electronic absorption spectra of complexes C3 and C8 (50 μM) in Tris/NaCl buffer (pH = 7.2) upon addition of CT-DNA (0–25 μM). Arrow shows the absorbance changing upon increase of DNA concentration. Inset: linear fit of [DNA]/(ɛa-ɛf) v/s [DNA] for the titration of the C3 and C8 complexes with CT-DNA
![Figure 11. Electronic absorption spectra of complexes C3 and C8 (50 μM) in Tris/NaCl buffer (pH = 7.2) upon addition of CT-DNA (0–25 μM). Arrow shows the absorbance changing upon increase of DNA concentration. Inset: linear fit of [DNA]/(ɛa-ɛf) v/s [DNA] for the titration of the C3 and C8 complexes with CT-DNA](/cms/asset/460e7781-3182-4e9f-b5b3-7c7f2cc890fe/teba_a_1758890_f0011_oc.jpg)
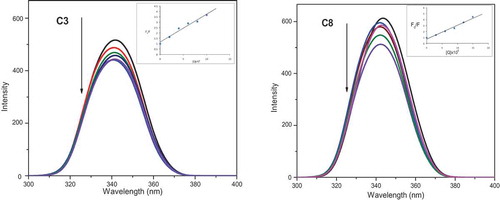
Figure 12. Emission spectra of EB bound to DNA in the presence of the complexes C3 and C8; λex = 450 nm, at the concentration 0–30 μM in Tris–HCl/NaCl buffer (pH 7.2). [EB] = 10 µM, [CT-DNA] = 100 µM. The arrow shows the intensity changes upon increasing concentrations of the complex. Inset: linear fit IO/I v/s [complex], Stern-Volmer quenching curves for the fluorescence titration of complexes C3 and C8 to DNA-EB bound system
![Figure 12. Emission spectra of EB bound to DNA in the presence of the complexes C3 and C8; λex = 450 nm, at the concentration 0–30 μM in Tris–HCl/NaCl buffer (pH 7.2). [EB] = 10 µM, [CT-DNA] = 100 µM. The arrow shows the intensity changes upon increasing concentrations of the complex. Inset: linear fit IO/I v/s [complex], Stern-Volmer quenching curves for the fluorescence titration of complexes C3 and C8 to DNA-EB bound system](/cms/asset/e1496495-09fa-41ae-8848-3f26c7339112/teba_a_1758890_f0012_oc.jpg)
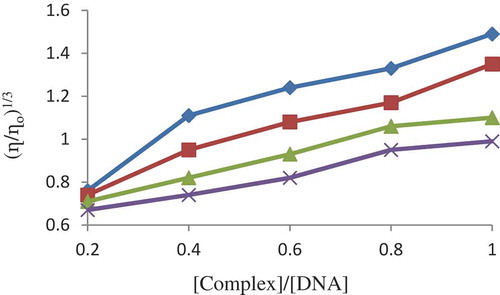
Figure 13. Effect of increasing the concentration of the complexes on the relative viscosities of CT‐DNA at 27.0 ± 0.1°C in 5 mM tris‐HCl buffer (pH = 7.2)