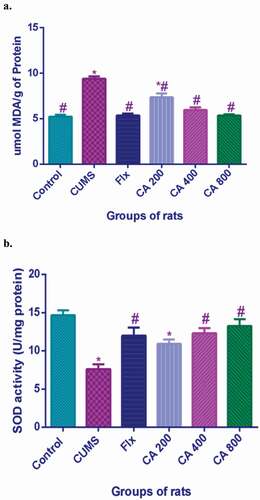
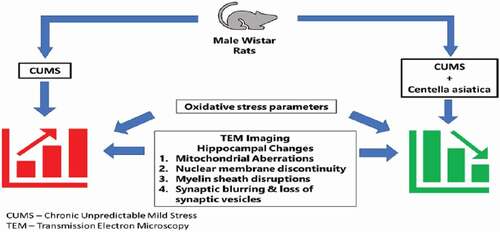
Figures & data
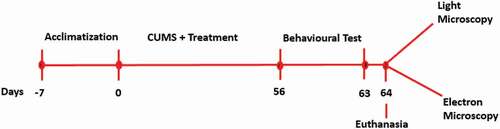
Table 1. Chronic unpredictable mild stress procedures.
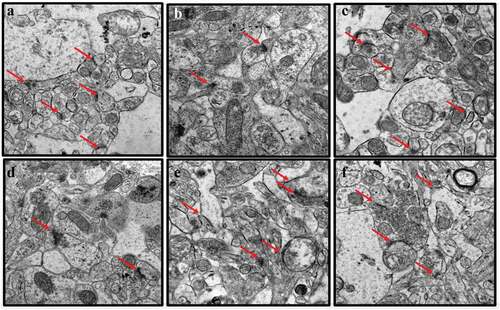
Figure 2. TEM images of rat’s brains showing mitochondrial aberrations in CUMS rats and those co-administered with fluoxetine and CA. a) Control group of rats showing normal mitochondria (blue arrow) with dense matrix, b) CUMS rats showing elongated and burst mitochondria (yellow arrow), c) fluoxetine group of rats showing normal mitochondria like those of the control group of rats, d) CA 200 group of rats showing normal mitochondria (blue arrow) and vacuolated mitochondria (red arrow), and e-f) CA 400 and 800 groups of rats, showing normal mitochondria.
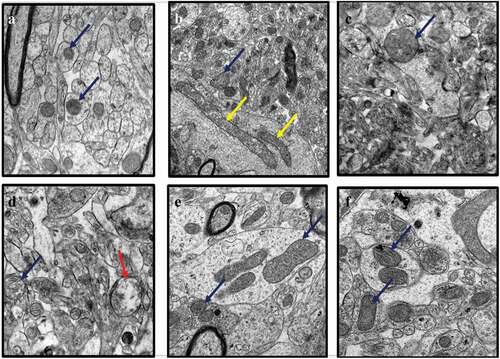
Figure 3. TEM micrographs of rat’s brains showing nuclear abnormalities in CUMS rats and those co-administered with Fluoxetine and CA. a) Control group of rats showing nucleoli (red arrow), evenly distributed chromatin and double nuclear membrane (blue arrow), b) CUMS rats showing pyknotic nucleus and crescent formation, c) fluoxetine group of rats showing normal nucleus with double nuclear membrane (blue arrow), d) CA 200 group of rats showing nucleus with degenerated chromatin and gaps in their nuclear membrane (purple arrow), e-f) CA 400 and 800 groups of rats with normal nucleus showing intact nuclear membrane (blue arrow) and nucleoli (red arrow).
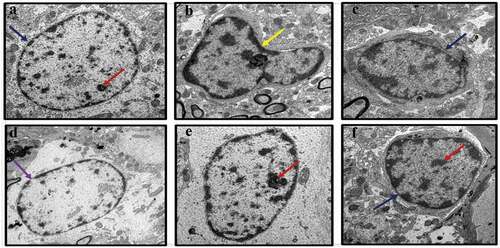
Figure 4. TEM micrographs of rat brains showing myelin sheath defects in CUMS rats and those co-administered with fluoxetine and CA. a) Control group of rats showing normal myelin sheaths appeared dense, thick and tightly wrapped around their axons (blue arrow). b) The CUMS rats showing discontinuous myelin (purple arrow) and onion-like bulging (red arrow) of their myelin sheaths. c) fluoxetine group of rats showed normal myelin sheaths. d) CA 200 group of rats showing distorted myelin sheaths (yellow arrow). e) CA 400 group of rats showing normal myelin sheath tightly wrapped around an axon (blue arrow) and f) CA 800 group of rats showing normal myelin sheath.
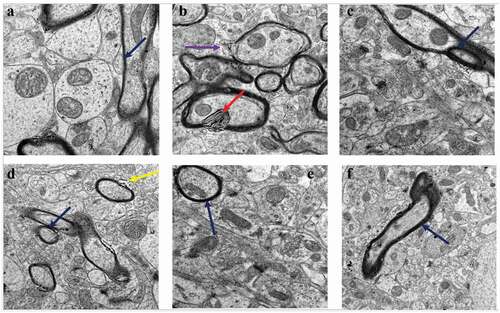
Figure 5. TEM micrographs of rat’s brains showing synaptic abnormalities in CUMS rats and those co-administered with fluoxetine and CA. a) Control group of rats showing abundant synapses (red arrows). b) CUMS rats showing few synapse. c) fluoxetine group of rats showing many normal looking synapses. d) CA 200 group of rats showing less numbers of synapses. e-f) CA 400 and 800 groups of rats showing abundant normal synapses.