Figures & data
Table 1. Vina search space used in this study.
Table 2. The 19 bioactive compounds that had drug-like characteristics, sorted by their log Po/w..
Table 3. Pharmacokinetics and metabolism prediction of bioactive compounds.
Figure 1. The toxicity evaluation and membrane permeability prediction of six potential compounds. a) Toxicity classification based on toxicity class and LD50. b) The probability of inducing several kinds of toxicity. c) The membrane permeability prediction of six bioactive compounds (top) with the energy required to pass the phospholipid membrane (bottom).
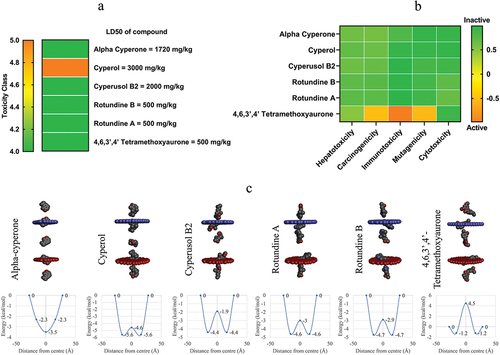
Figure 2. The visualization of molecular docking between α-bungarotoxin (cyan) with a) Alpha-cyperone, b) Cyperol, c) Cyperusol B2, d) Rotundine a, e) Rotundine b, f) 4,6,3’,4’-tetramethoxyaurone, g) Anti-α-bungarotoxin peptide. Dashed lines represent interaction type, with the dark and light green lines representing hydrogen bonds and the pink line representing hydrophobic interaction.
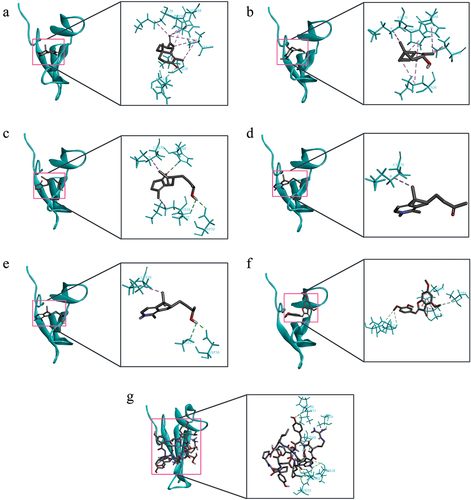
Table 4. Detailed interactions between α-bungarotoxin with bioactive compounds and inhibitor control.
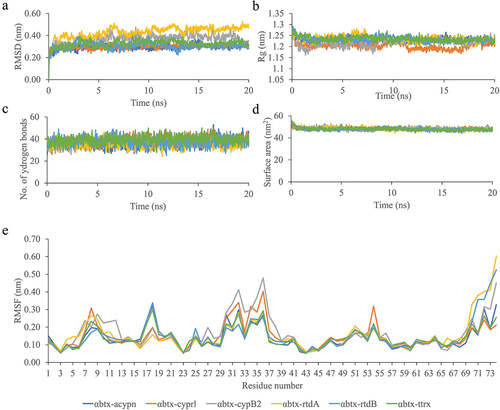
Figure 3. The RMSD (a), Radius of gyration (b), Number of Hydrogen Bond (c), Surface area (d), and RMSF (e) Calculation for α-bungarotoxin and bioactive compound complexes after 20 nanoseconds of simulation. αbtx-acypn: α-bungarotoxin- alpha-cyperone, αbtx-cyprl: α-bungarotoxin-cyperol, αbtx-cypB2: α-bungarotoxin-cyperusol B2, αbtx-rtdA: α-bungarotoxin-rotundine A, αbtx-rtdB: α-bungarotoxin-rotundine B, αbtx-ttrx: α-bungarotoxin-4,6,3’,4’-tetramethoxyaurone.