Figures & data
Table 1. Mineralogy and particle size of raw materials
Table 2. Sample designation
Table 3. XRF analyses of raw materials
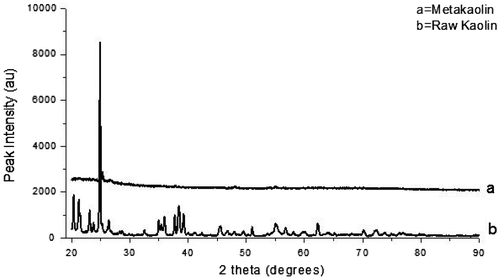
Figure 2. The XRD pattern for the samples crystallized for 3 h (for batch formulations containing bauxite).
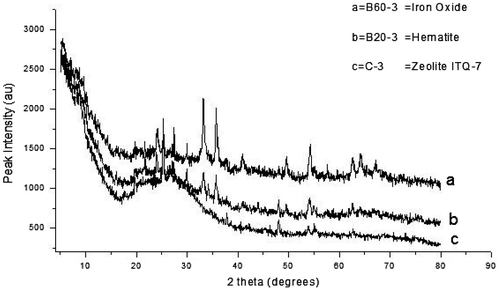
Figure 3. XRD pattern of the control experiment with the as-synthesized zeolite varied with bauxite addition.
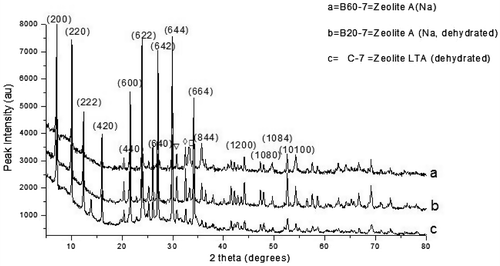
Figure 4. XRD pattern of the control experiment with the as-synthesized zeolite varied with feldspar addition.
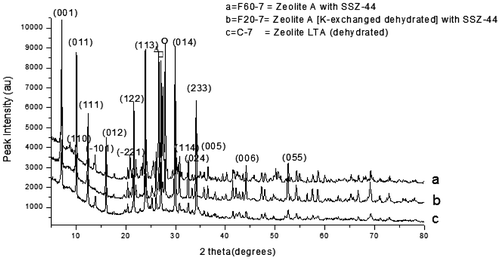
Figure 5. XRD pattern of the control experiment with the as-synthesized zeolite varied with raw silica addition.
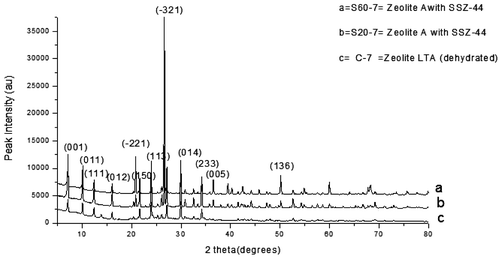
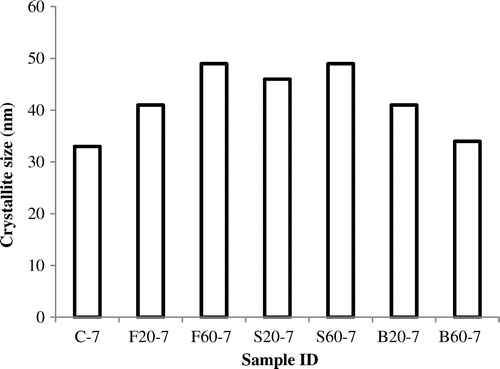
Table 4. Process conditions and XRD results of synthetic zeolites
Figure 7. The FTIR of the control experiment and the synthesized zeolite samples containing 20 wt.% of bauxite, feldspar and silica respectively.
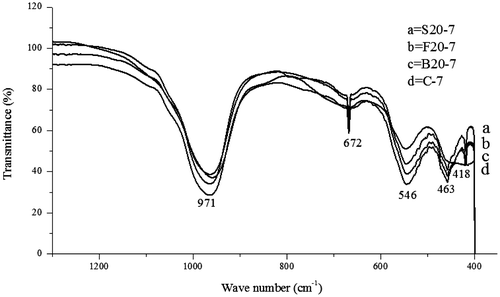
Figure 8. The FTIR of the control experiment and the synthesized zeolite samples containing 60 wt.% of bauxite, feldspar and silica, respectively.
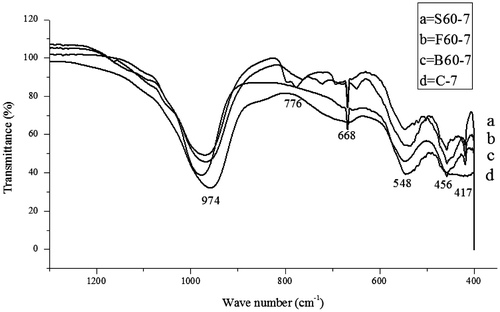
Figure 9. The N2 adsorption/desorption isotherm of synthesized zeolite (Control experiment, C-7) nanoparticles.
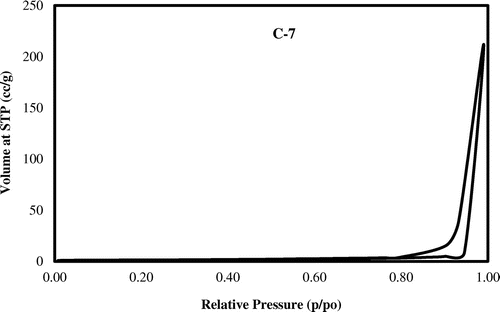
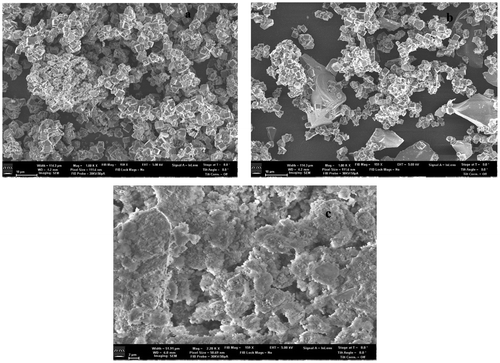
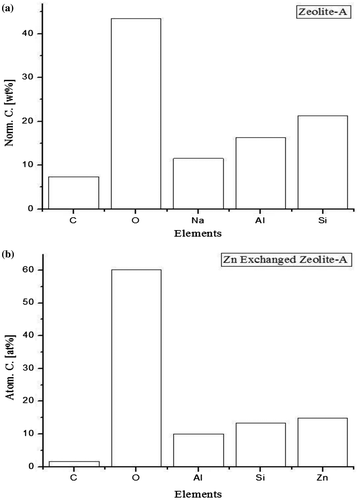
Figure 10. SEM of (a) Zeolite A; (b) SEM of Zeolite A formulated with 60% Silica; (c) SEM of Zn exchanged Zeolite A.