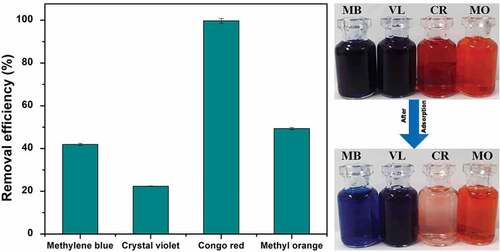
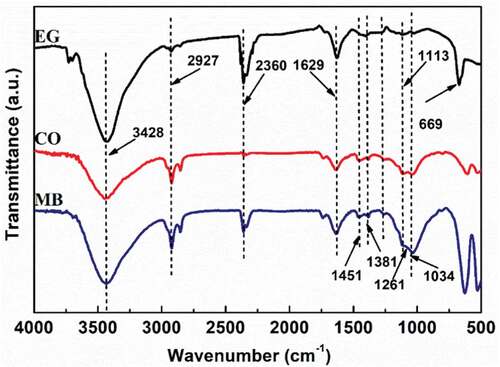
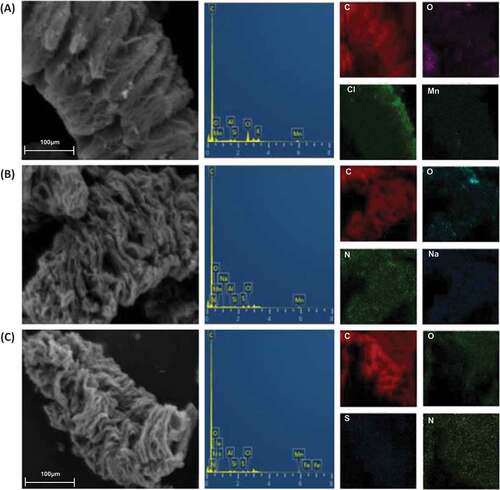
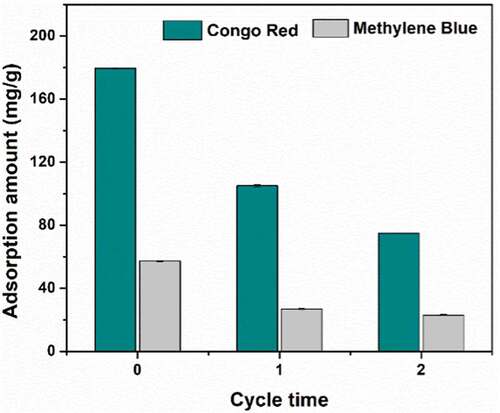
Figures & data
Figure 3. XRD spectra of graphite and expanded graphite. The shift of (0 0 2) peak was observed in the inset image
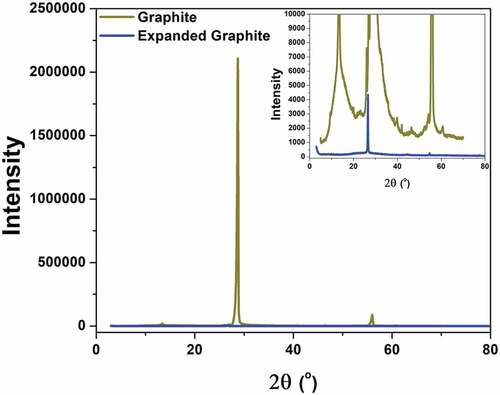
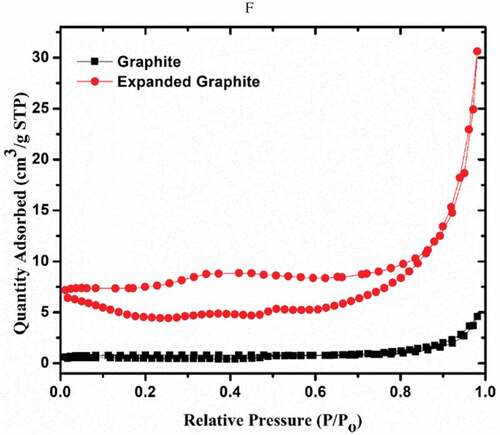
Table 1. Pore parameters of the graphite-based adsorbents
Figure 6. The effect of the contact time for EG with the adsorption capacity (a) and the removed efficiency (b)
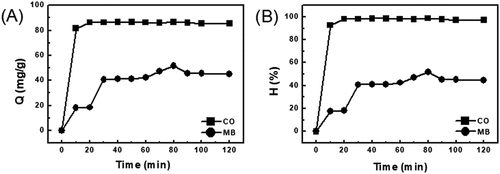
Figure 7. Effect of pH in the aqueous solution for EG with the adsorption capacity (a) and the removed efficiency (b)
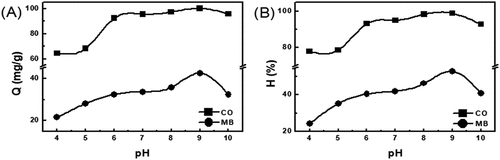
Figure 8. The effect of amount of adsorbent of EG with the adsorption capacity (a) and the removed efficiency (b)
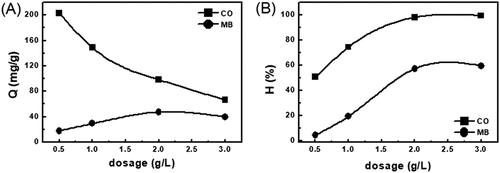
Figure 9. Effect of the initial concentration of Congo red and methylene blue on EG’s adsorption capacity (a) and removed efficiency (b)
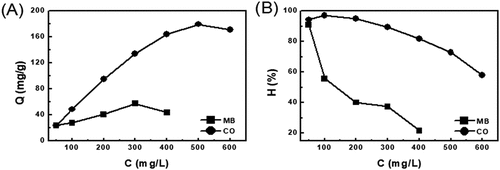
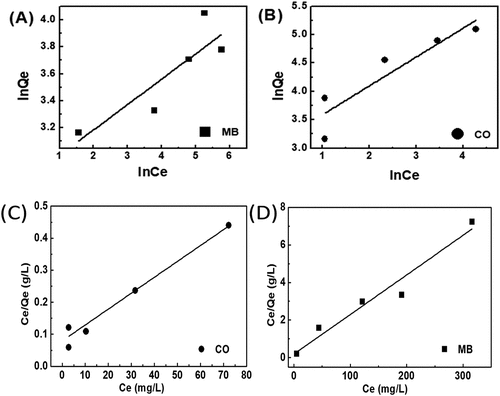
Table 2. Langmuir and Freundlich isotherm model constants for adsorption of CO and MB
Figure 10. Freundlich (a,b) model and Langmuir (c,d) and of the dye adsorption onto EG with Congo red and methylene blue