Figures & data
Figure 2. (a) SEM images of bare CuO nanoparticles with different magnification. (b) SEM images of EDTA functionalized CuO nanoparticles. (c) SEM images of EDTA-silane functionalized CuO nanoparticles.
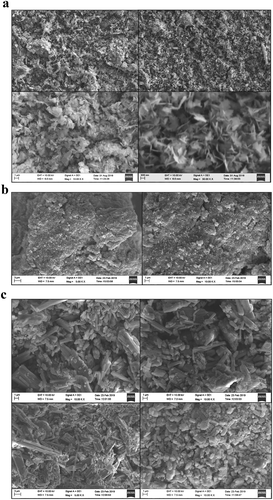
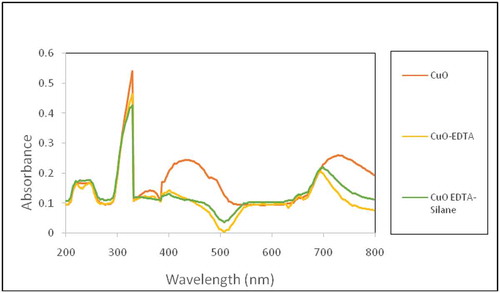
Figure 3. FTIR spectra showing the comparison between the peaks of pure and functionalized CuO nanoparticles.
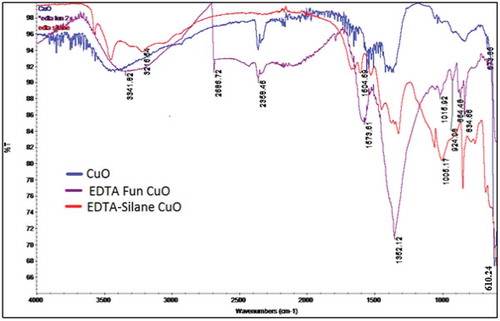
Figure 4. (a) XPS spectra of EDTA functionalized CuO nanoparticles showing the presence of C by ejection of electron from C-1 s orbital. (b) XPS spectra of EDTA functionalized CuO nanoparticles showing the presence of Cu by the ejection of electron from the Cu-2p orbital. (c) XPS spectra of EDTA functionalized CuO nanoparticles showing the presence of Cu by the ejection of electron from the O-1 s orbital.
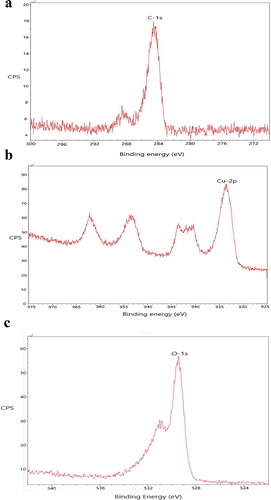
Figure 5. (a) BET adsorption isotherm showing the relation between volume and relative pressure giving surface area of the material. (b) BET adsorption isotherm showing the relation between differential pore volume and pore width giving pore diameter of the nanoparticle.
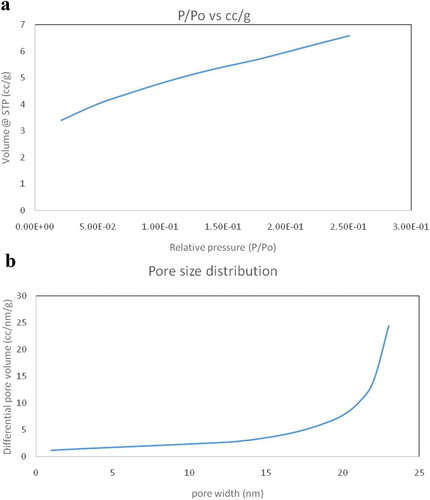
Table 1. Percentage adsorption by nanoparticles