Figures & data
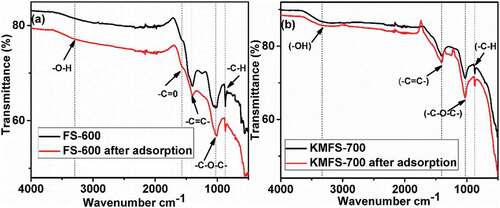
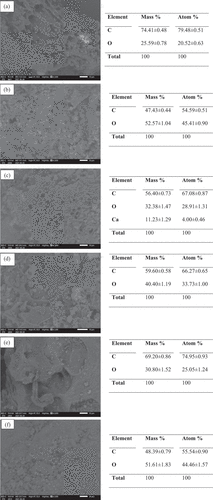
Figure 3. SEM and EDX results for (a) FS-500, (b) FS-600, (c) FS-700, (d) KMFS-500, (e) KMFS-600 and (f) KMFS-700.
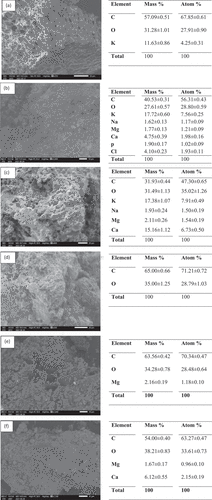
Table 1. Point zero charge pH(PZC) of the adsorbent
Figure 5. Effect of concentration for the removal of Cu(II) on (a) FS-500, FS-600 and FS-700; (b) Cr(VI) on FS-500, FS-600; (c) Cu(II) on KMFS-500, KMFS-600 and KMFS-700 and Cr(VI) (d) KMFS-500, KMFS-600 and KMFS-700.[Conditions: pH(5), adsorbent dosage (0.1 g), contact time (2 h), solution volume (20 mL), temperature (298 K) and agitation speed(200 rpm)].
![Figure 5. Effect of concentration for the removal of Cu(II) on (a) FS-500, FS-600 and FS-700; (b) Cr(VI) on FS-500, FS-600; (c) Cu(II) on KMFS-500, KMFS-600 and KMFS-700 and Cr(VI) (d) KMFS-500, KMFS-600 and KMFS-700.[Conditions: pH(5), adsorbent dosage (0.1 g), contact time (2 h), solution volume (20 mL), temperature (298 K) and agitation speed(200 rpm)].](/cms/asset/72a01297-1413-4a44-9f63-54655c6109c9/oaen_a_2119530_f0005_oc.jpg)
Figure 6. Effect of time for the removal of Cu(II) on (a) FS-500, FS-600 and FS-700; (b) Cr(VI) on FS-500, FS-600; (c) Cu(II) on KMFS-500, KMFS-600 and KMFS-700 and Cr(VI) (d) KMFS-500, KMFS-600 and KMFS-700. [Conditions: pH(5), adsorbent dosage (0.1 g), concentration (100 mg/L, solution volume (20 mL), temperature (298 K) and agitation speed(200 rpm)].
![Figure 6. Effect of time for the removal of Cu(II) on (a) FS-500, FS-600 and FS-700; (b) Cr(VI) on FS-500, FS-600; (c) Cu(II) on KMFS-500, KMFS-600 and KMFS-700 and Cr(VI) (d) KMFS-500, KMFS-600 and KMFS-700. [Conditions: pH(5), adsorbent dosage (0.1 g), concentration (100 mg/L, solution volume (20 mL), temperature (298 K) and agitation speed(200 rpm)].](/cms/asset/8dbadc0e-6c38-4d32-bb96-bd385fe857e8/oaen_a_2119530_f0006_oc.jpg)
Figure 8. Effect of pH for the removal of Cu(II) on (a) FS-500, FS-600 and FS-700; (b) Cr(VI) on FS-500, FS-600; (c) Cu(II) on KMFS-500, KMFS-600 and KMFS-700 and Cr(VI) (d) KMFS- 500, KMFS-600 and KMFS-700. [Conditions: temperature (298 K), adsorbent dosage (0.1 g), concentration (100 mg/L, solution volume (20 mL), time (2 h) and agitation speed (200 rpm)].
![Figure 8. Effect of pH for the removal of Cu(II) on (a) FS-500, FS-600 and FS-700; (b) Cr(VI) on FS-500, FS-600; (c) Cu(II) on KMFS-500, KMFS-600 and KMFS-700 and Cr(VI) (d) KMFS- 500, KMFS-600 and KMFS-700. [Conditions: temperature (298 K), adsorbent dosage (0.1 g), concentration (100 mg/L, solution volume (20 mL), time (2 h) and agitation speed (200 rpm)].](/cms/asset/cb401487-6c43-474e-9f60-f4955ecd7116/oaen_a_2119530_f0008_oc.jpg)
Table 2. Adsorption kinetics studies for carbonized fennel seeds
Table 3. Adsorption kinetics studies for KMnO4 treated carbonized fennel seeds
Table 4. Adsorption isotherms studies for carbonized fennel seeds
Table 5. Adsorption isotherms studies for KMnO4 treated carbonized fennel seeds
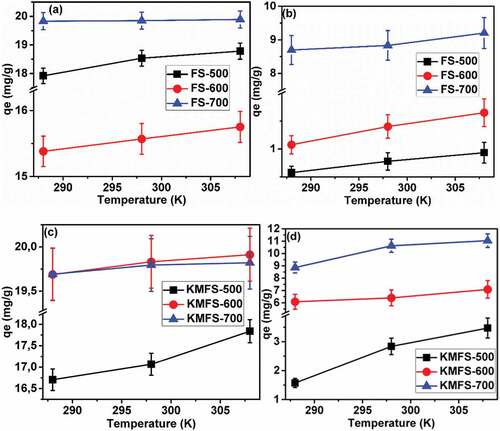
Table 6. Adsorption thermodynamic studies for carbonized fennel seeds
Table 7. Comparison with other adsorbents
Figure 9. Reusability studies for (a) Cu(II) and (b) Cr(VI) on carbonized fennel seeds and (c) Cu(II) and (d) Cr(VI) on KMnO4 treated carbonized fennel seeds. Mechanism for Cr(VI) and Cu(II).
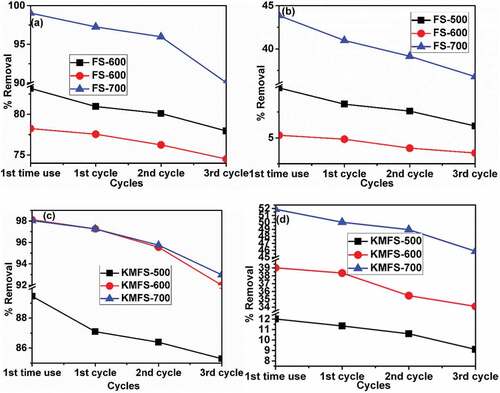
Figure 10. Schematic diagram for the adsorption mechanism of Cr(VI) and Cu(II) on the adsorbents surface.
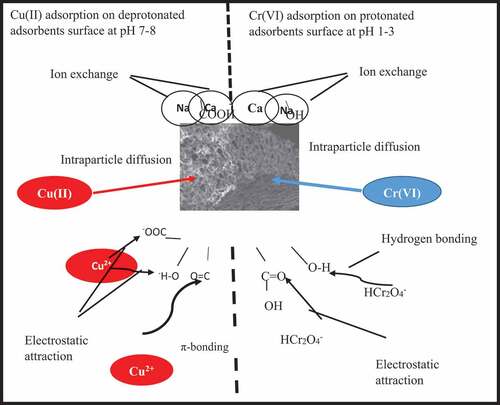
Figure 12. SEM images and EDX results for (a) FS-500, (b) FS-600, (c) FS-700. (d) KMFS-500, (e) KMFS-600 and (f) KMFS-700 after adsorption.
Data availability statement
The data supporting the findings of the study may be made available from the corresponding authors on request.