Figures & data
Figure 1. Cyclic voltammograms of 1.0 mM K3[Fe(CN)6] in 1.0 M KCl at different scan rates: (a) 0.003; (b) 0.005; (c) 0.007; (d) 0.01; (e) 0.03; (f) 0.05; (g) 0.09; (h) 0.1 V s−1. Inner figure represents the dependence of peak current Ip/μA on the square root of scan rate υ1/2/V s−1.
![Figure 1. Cyclic voltammograms of 1.0 mM K3[Fe(CN)6] in 1.0 M KCl at different scan rates: (a) 0.003; (b) 0.005; (c) 0.007; (d) 0.01; (e) 0.03; (f) 0.05; (g) 0.09; (h) 0.1 V s−1. Inner figure represents the dependence of peak current Ip/μA on the square root of scan rate υ1/2/V s−1.](/cms/asset/45c84f99-6318-4215-9d8f-2bf13ea89cb2/oach_a_1153274_f0001_oc.gif)
Figure 2. Cyclic voltammograms of 1.0 m M MDH on GCE in pH 9.2, phosphate buffer (I = 0.2 M) (a) blank and (b) MDH run at 0.1 V s−1.
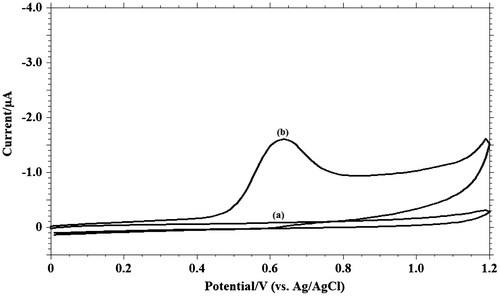
Figure 3. Surfactant behaviour on MDH: Cyclic voltammograms of MDH (a) with cetyltrimethylammonium bromide (anionic surfactant); (b) with sodium dodecyl sulfate (cationic surfactant); (c) with Triton-X (non-ionic surfactant); (d) without surfactant.
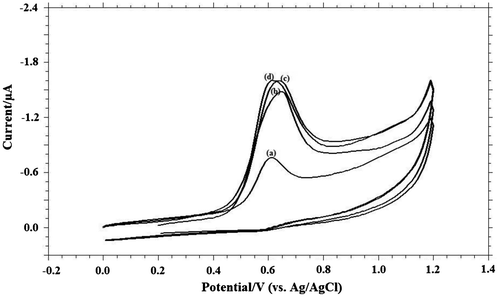
Figure 4. (A) Cyclic voltammograms obtained for 1.0 mM MDH in buffer solution at (a) blank; (b) pH 6.0; (c) pH 8.0; (d) pH 9.2; (e) pH 10.4; (f) pH 11.2; (B) Variation of peak currents Ip / μA of MDH with pH; (C) Influence of pH on the peak potential Ep/V of MDH.
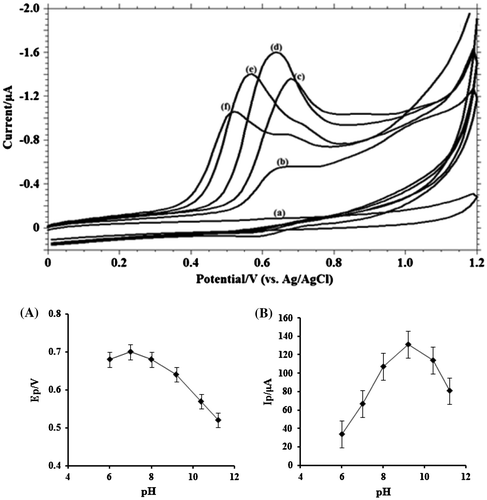
Figure 5. Cyclic voltammograms of 1.0 mM MDH in buffer solution of pH 9.2 (I = 0.2 M) at scan rate of: (1) blank; (2) 0.001; (3) 0.005; (4) 0.01; (5) 0.02; (6) 0.03; (7) 0.05; (8) 0.06; (9) 0.08; (10) 0.1; (11) 0.13; (12) 0.17; (13) 0.20; (14) 0.30 V s−1. (A) Dependence of peak current (Ip/μA) on the scan rate (υ/V s−1); (B) Plot of logarithm of peak current (log Ip/μA) vs. logarithm of scan rate (log υ/V s−1); (C) Plot of variation of peak potential (Ep/V) with logarithm of scan rate (log υ/V s−1).
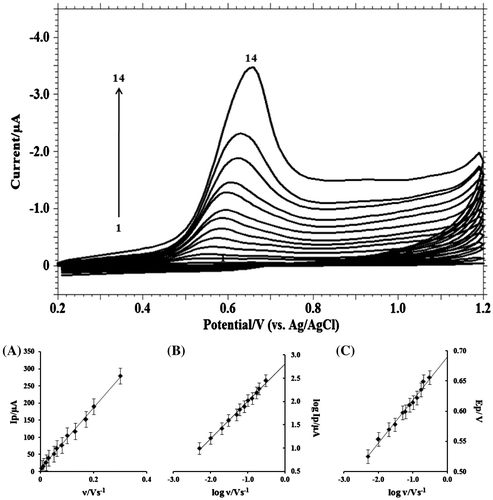
Figure 6. Linear sweep voltammograms of 1.0 mM MDH in 0.2 M phosphate buffer solution of pH 9.2 at scan rate of: (1) blank; (2) 0.001; (3) 0.002; (4) 0.004; (5) 0.008; (6) 0.01; (7) 0.02; (8) 0.04; (9) 0.06; (10) 0.08; (11) 0.15; (12) 0.20; (13) 0.30 V s−1.
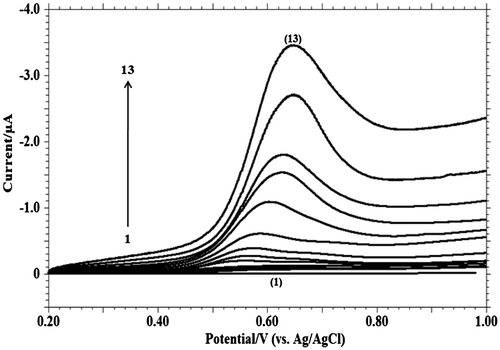
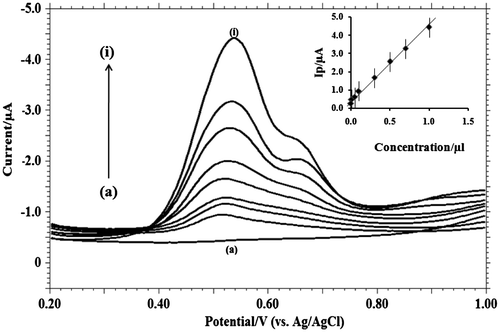
Figure 7. Differential-pulse voltammograms with increasing concentrations of MDH in pH 9.2 phosphate buffer solution on GCE (a) blank; (b) 1 × 10−3; (c) 3 × 10−3; (d) 5 × 10−3; (e) 7 × 10−3; (f) 1 × 10−4; (g) 1 × 10−5; (h) 5 × 10−5; (i) 3 × 10−6. Inner figure represents the plot of peak current (Ip/μA) vs. concentration (C).