Figures & data
Figure 2. Variation of weight loss with time for the corrosion of mild steel in 0.1 M H2SO4 containing various concentrations of 3-nitrobenzoic acid at 303, 313, 323 and 333 K.
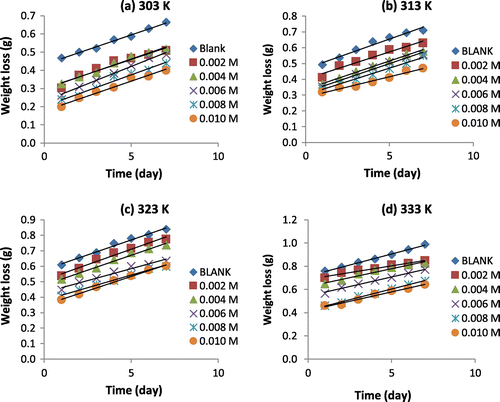
Table 1. Corrosion rate of mild steel (in solution of 0.1 M H2SO4) and inhibition efficiency of 3-nitrobenzoic acid for mild steel corrosion (in 0.1 M H2SO4)
Table 2. Polarization data for the corrosion of mild steel in 0.1 M H2SO4 in the absence and presence of 3-nitrobenzoic acid at 303 K
Figure 3. Potentiodynamic polarization curves for the mild steel in 0.1 M H2SO4 in the absence and presence of different concentrations of 3-nitrobenzoic acid.
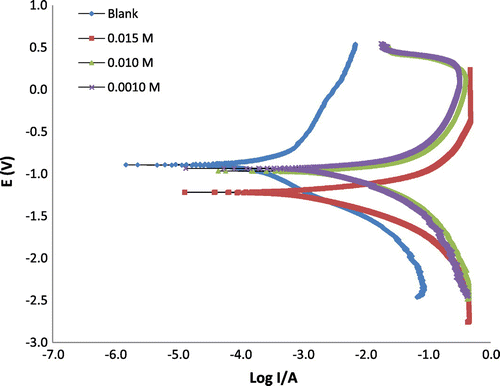
Figure 4. Nyquist plots for the mild steel in 0. 1 M H2SO4 in the absence and presence of different concentrations of 3-nitrobenzoic acid.
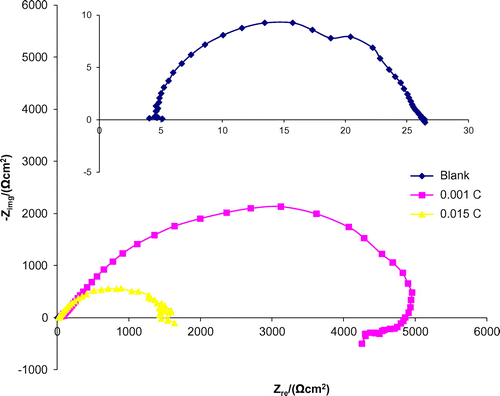
Table 3. EIS parameters for corrosion of mild steel in 0.1 M H2SO4 in the absence and presence of different concentrations of 3-nitrobenzoic acid at 303 K
Figure 5. Arrhenius plot for the corrosion of mild steel in 0.1 M H2SO4 containing various concentrations of 3-nitrobenzoic acid.
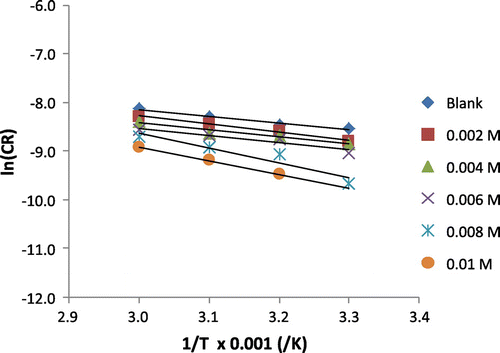
Table 4. Arrhenius and Transition state parameters for the adsorption of 3-nitrobenzoic acid on mild steel surface
Figure 6. Transition state plot for the corrosion of mild steel in 0.1 M H2SO4 containing various concentrations of 3-nitrobenzoic acid.
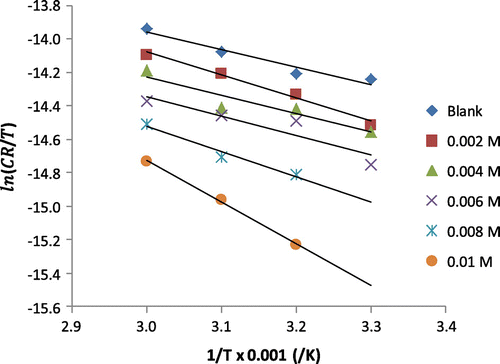
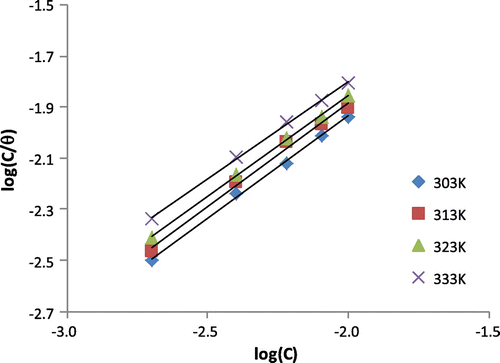
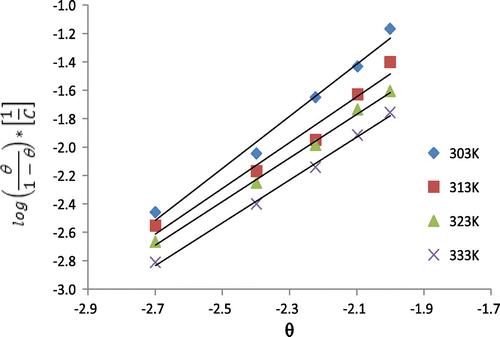
Table 5. Langmuir and Frumkin parameters for the adsorption of 3-nitrobenzoic acid on the surface of mild steel
Figure 9. Scanning electron micrographs of the corrosion product of mild steel in the absence and presence of 3-nitrobenzoic acid (as an inhibitor) at various magnifications.