Figures & data
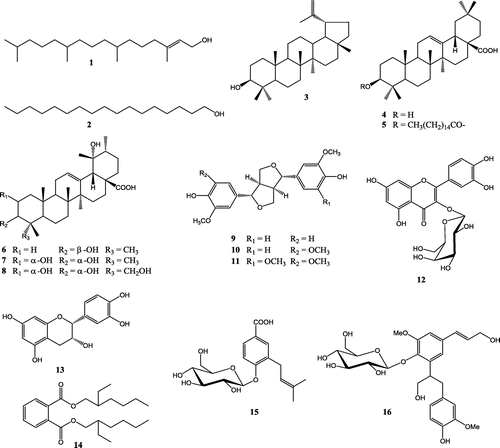
Figure 1. Chemical structures of isolated compounds (1–16): (E)-phytol (1), heptadecan-1-ol (2), lupeol (3), oleanolic acid (4), 3β-hexadecanoyloleanolic acid (5), pomolic acid (6), euscaphic acid (7), myrianthic acid (8), (+)-pinoresinol (9), (+)-medioresinol (10), (+)-syringaresinol (11), quercetin-3-O-β-D-galactopyranoside (12), epicatechin (13), bis-(2-ethylhexyl) phthalate (14), 3-prenyl-4-O-β-D-glucopyranosyloxy-4-hydroxylbenzoic acid (15), icariside E5 (16).