Figures & data
Table 1. Physicochemical properties of WAC-nZVI nanocomposites
Table 2. Important FTIR bands of WAC-nZVI with their possible functional groups before and after Pb2+ adsorption
Figure 4. The effect of pH on Pb2+ adsorbed onto WAC-nZVI.
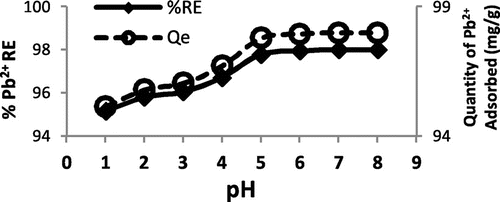
Figure 5. Effect of WAC-nZVI dose on Pb2+ adsorbed.
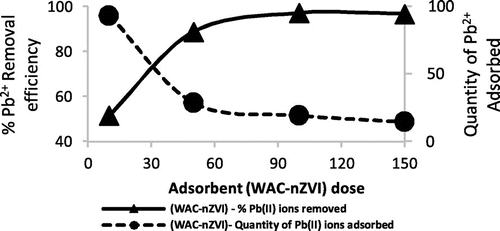
Figure 6. Effect of contact time on Pb2+ adsorbed onto WAC-nZVI .
Figure 7. (a–d): Linear plots of (a) Pseudo-first-order, (b) Pseudo-second-order, (c) Elovich, (d) Fractional power.
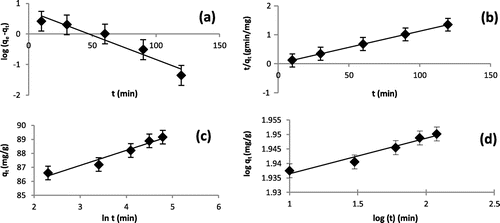
Table 3. Adsorption kinetic models’ parameters for the sorption of Pb2+ onto WAC-nZVI
Figure 8. (a–d) Linear plots of (a) Intraparticle Diffusion, (b) External Diffusion, (c) Bangham, and (d) Boyd mechanism models for adsorption of Pb2+ onto WAC-nZVI.
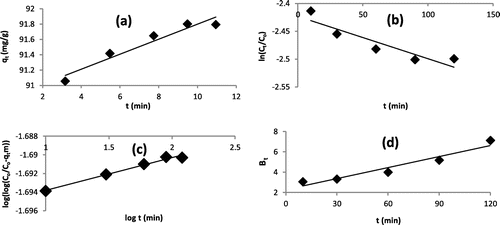
Table 4. Adsorption mechanism models for immobilization of Pb2+ onto WAC-nZVI
Figure 9. Percentage and quantity of Pb2+ adsorbed onto WAC-nZVI at various initial Pb2+concentrations.
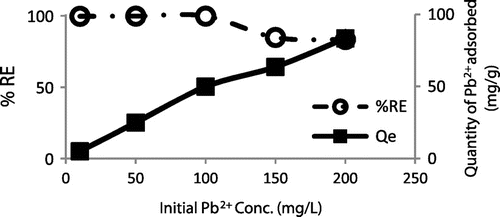
Table 5. Different adsorption isotherm models (Citation15, 21, 44–46)
Figure 10. (a–e): Linear plots of (a) Freundlich, (b) Langmuir, (c) Temkin, (d) D-R, (e) Halsey (f) Harkin–Jura, (g) Jovanovic isotherm models for sorption of Pb2+ onto WAC-nZVI, and (h) Plot of Langmuir dimensionless separation factor for adsorption of Pb2+ onto WAC-nZVI.
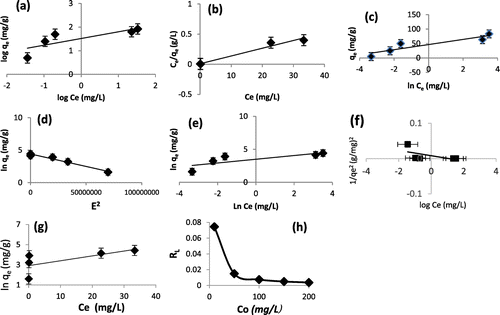
Table 6. Isotherm models’ parameters and evaluated values for adsorption of Pb2+ onto WAC-nZVI
Table 7. Comparison of the previously reported adsorbents used in Pb2+ uptake with the present ones under investigation
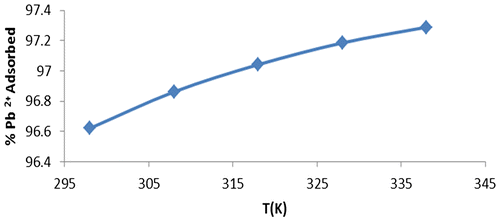
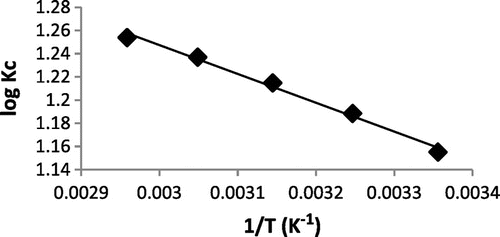
Table 8. Thermodynamic parameters for adsorption of Pb2+ onto WAC-nZVI
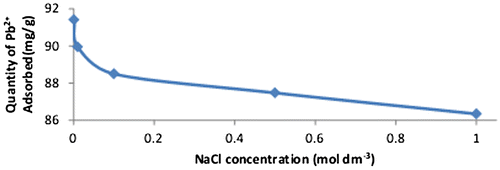
Figure 13. Ionic strength on Pb2+ adsorbed onto WAC-nZVI.