Figures & data
Table 1. Different substituents of aralkyl/phenyl/aryl group
Table 2. AChE inhibition studies
Table 3. Anti-urease activity
Figure 1. Obtained binding modes of ligands in the main binding site of Acetylcholinesterase. (A) Superimposing of compounds 6p (Purple), 6a (Green) and 6e (Yellow) docked to AChE. (B) Binding mode of compound 6p in AChE main binding site. (C) Binding mode of compound 6e in AChE main binding site. (D) Binding mode of compound 6a in AChE main binding site.
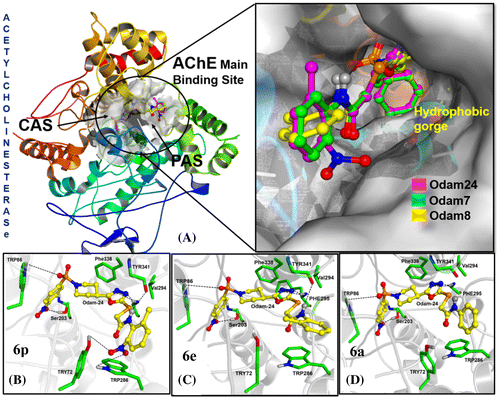
Table 4. Binding constant and number of binding site of compounds warfarin and ibuprofen with BSA at 298 K
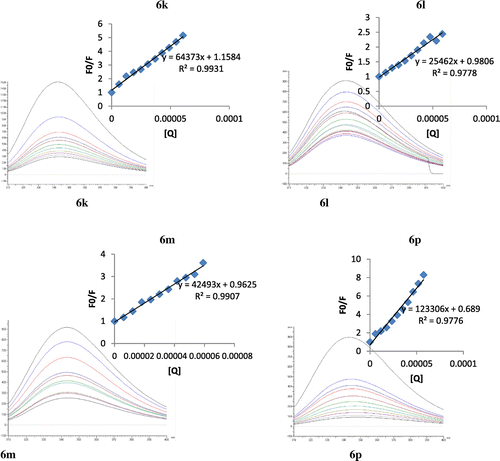
Figure 2. Fluorescence spectra of BSA in presence of different amounts of selected compounds (6k, 6l, 6m, and 6p) at 295 nm and 298 K (inset graph corresponding to the Stern-Volmer plot).
Scheme 1. General scheme for the synthesis of 2-(5-(1-(4-nitrophenylsulfonyl)piperidin-4-yl)-1,3,4-oxadiazol-2-ylthio)-N-(substituted)acetamide (6a–p). (A) 15% Na2CO3 solution, H2O, stir for 8 h. (B) N2H4.H2O, EtOH, reflux for 5 h. (C) KOH (s), EtOH, reflux for 6 h. (D) BrCH2COBr, 15% Na2CO3 solution, H2O, stir for 2 h. (E) DMF, NaH, stir for 24 h.