Figures & data
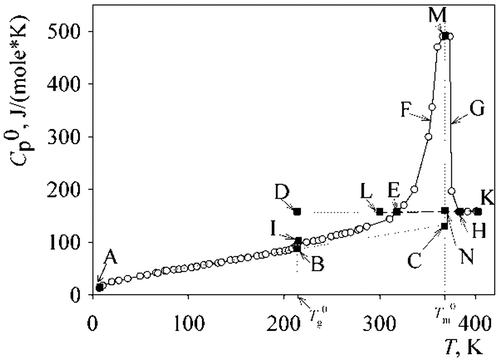
Figure 2. Temperature dependence of the PAL heat capacity. (Mr∼1300. ABC, crystalline; AIE, partly crystalline; AB, vitreous; DEN, high elastic; EMH, apparent melting heat capacity; ENK, liquid; EL, metastable liquid).
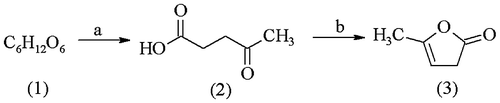
Scheme 1. Synthesis of α-angelicalactone (3). Reaction conditions: (a) HCl (cat.), 370–373 K, 4 h; (b) H3PO4 (cat.), 25 Torr, 340–345 K.