Figures & data
Figure 1. Chromatogram of a single injection of solution containing standards of sulfadoxine and pyrimethamine in a binary mixture.
Sulf—sulfadoxine, Pyr—pyrimethamine.
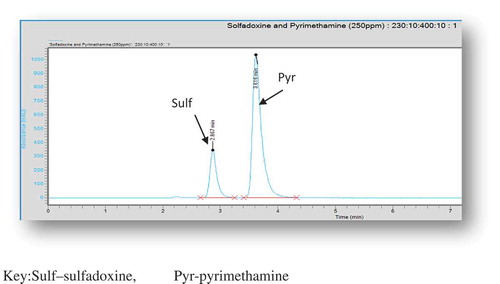
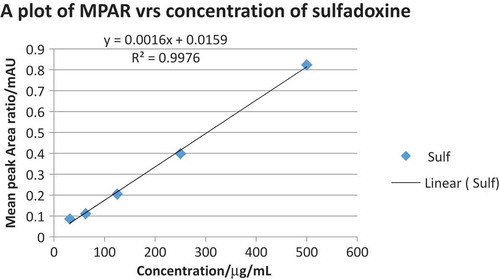
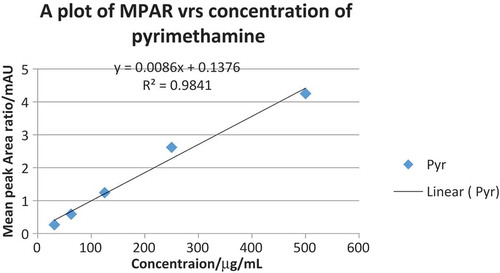
Table 1. Calibration curve data for sulfadoxine and pyrimethamine
Table 2. Mean recovery of sulfadoxine and pyrimethamine from binary mixture
Table 3. Summary of validation parameters of sulfadoxine and pyrimethamine
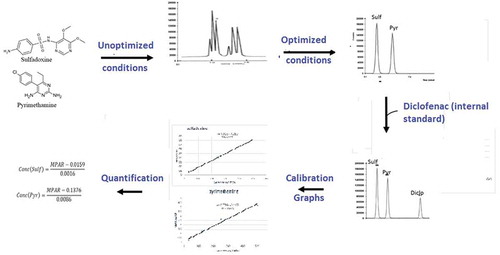
Figure 2. Chromatogram of a single injection of solution containing standards of sulfadoxine, pyrimethamine and diclofenac (internal standard).
Sulf—sulfadoxine, Pyr—pyrimethamine, Diclo—diclofenac.
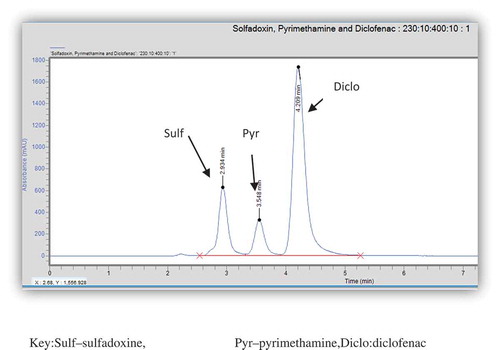
Table 4. Percent purity of sulfadoxine and pyrimethamine in tablet dosage form using external standard
Table 5. Content of sulfadoxine and pyrimethamine in tablet dosage form using internal standard