Figures & data
Figure 2. Effect of adsorbent particle sizes on adsorption of Cd, Cr and Pb onto E. camaldulensis activated carbon (EACA) (dose = 1 g; pH = 7; Time = 2 h; concentration = 0.25 mg/L in 100 mL volume of solution at room temperature)
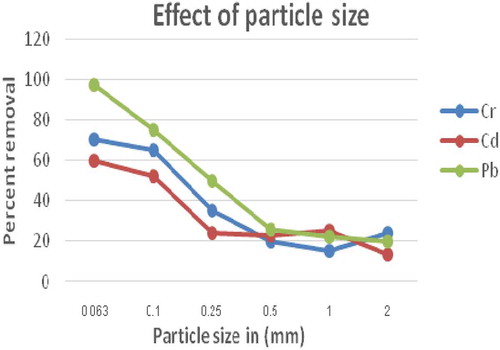
Figure 3. Effect of adsorbent dosage of E. camaldulensis activated carbon on to Cr, Cd and Pb (Concentration 0.25 mg/L, pH = 10.0 for Cd and Cr, pH = 12.0 for Pb, 0.063 mm adsorbent particle size and 2 h)
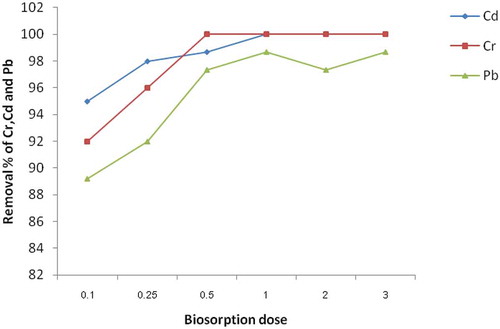
Figure 4. The effect of contact time on removal of Cr, Cd & Pb using E. camaldulensis activated carbon (Size 0.063 mm; dose = 1 g; pH = 7; concentration = 0.25 mg/L in 100 mL volume of solution at room temperature)
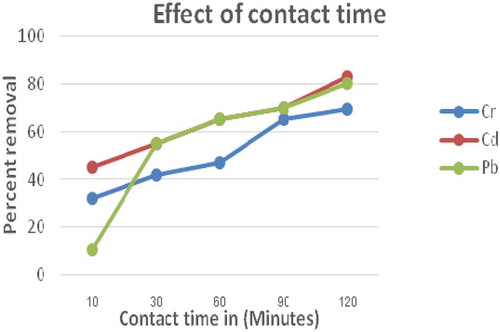
Figure 5. Effect of pH on biosorption of Pb, Cr and Cd using E. camaldulensis activated carbon (Size = 0.063 mm; dose = 1 g; Time = 2 h; concentration = 0.25 mg/L in 100 mL volume of solution at room temperature)
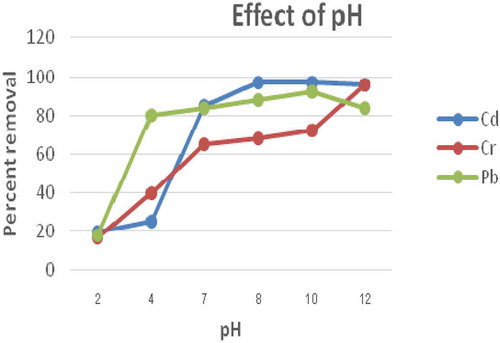
Figure 6. Effect of initial concentration on biosorption of Pb, Cd and Cr using E. camaldulensis activated carbon (Size = 0.063 mm, Time = 2 h, pH = 10 for Pb and Cr and 12 for Cd, dose = 1g)
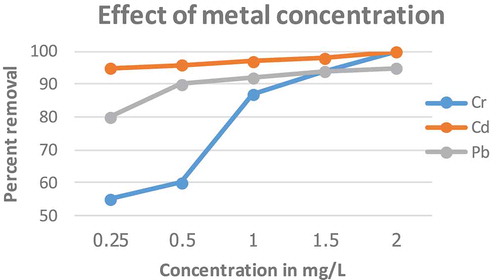
Table 1. Adsorption isotherms constants for Pb, Cr and Cd
Table 2. Comparison of the maximum adsorption capacity of Eucalyptus activated carbon with different studied commercial activated carbon as adsorbents
Table 3. Results of preliminary biosorption experiments for total Pb, Cd and Cr (bio-sorbent dose = 1 g, pH (Pb and Cd = 10: Cr = 12), contact time = 2h) from real sample
Table 4. Column adsorption of Cr, Pb, and Cd at 11g, pH of 10, 2 h wastewater at 5 and10 ml/min of flow rate
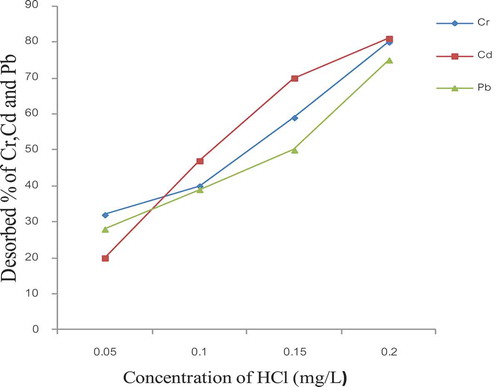
Figure 7. Desorption of Cr, Cd and Pb by HCl from E. camaldulensis activated carbon (condition 11g, 2 h and 200 rpm)