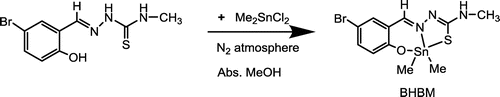Figures & data
Figure 1. (A) Synthesis of dimethyl tin(IV): 5-bromo-2-hydroxy benzaldehyde-N(4)-methyl thiosemicarbazone; (B) IR spectrum; (C) UV-visible spectrum of dimethyltin(IV)-N(4)-methylthiosemicarbazone complex; (D) 1H NMR spectrum; (E) 13C NMR spectrum.
Figure 2. The compound screening procedure: first, synthesized compound was tested in three cancer cell lines, MCF7, HCT116, A549, and normal cell lines EAhy926, at multiple concentrations of 6.25 to 200 μg/ml.
Figure 3. Photomicrographic images of different cancer cell lines and normal endothelial cells treated with BHBM tamoxifen, and 5-fluoruracil (5FU).
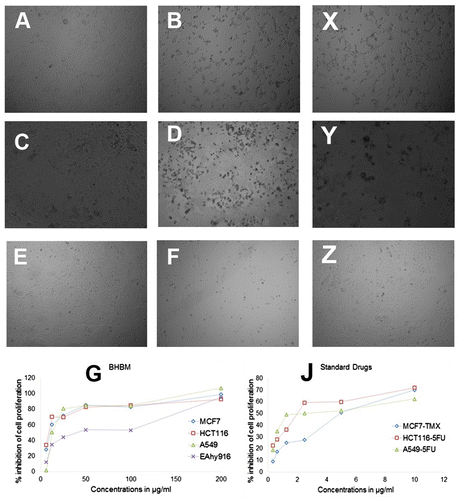
Table 1. IC50 and selectivity index (SI) of the various melanoma (cancer) cells
Figure 4. Antimetastatic functional assay: migration.
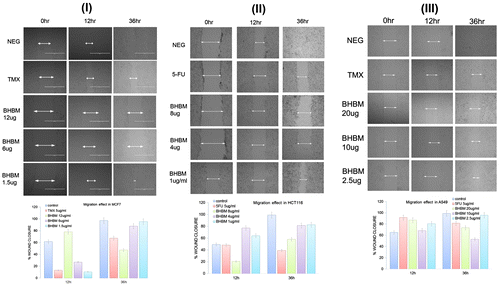
Figure 5. BHBM at 1-12 μg/ml negatively influenced the clonogenic growth of melanoma cells.
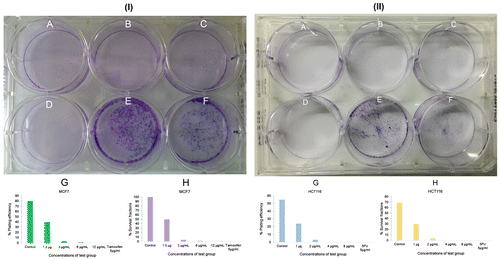
Figure 6. Three-dimensional (3D) tumor spheroid-based functional assay: invasion. 48-well agar-coated flat-bottomed plates were used to generate A549 spheroids (a single spheroid per well).
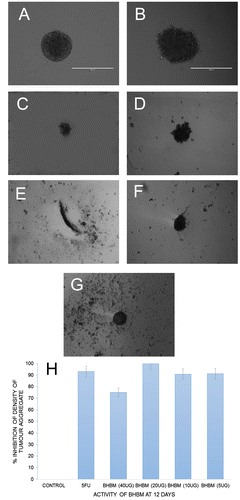
Figure 7. Photomicrographic images of the MCF7 in nuclear condensation assay (I). Staining of cultures by the use of Hoechst revealed the damaged DNA as an indicator of apoptosis. Higher cell death was found at 24 h (data not shown) than the tamoxifen. BHBM showed almost similar apoptotic index with mid and high doses at 12 h. The medium dose 6 μg/ml was more effective than the higher dose. BHBM at very high dose was not pronounced as anticancer activity. Values were determined as means ± SD. Photomicrographic images of the MCF7 in mitochondrial membrane potential (II).
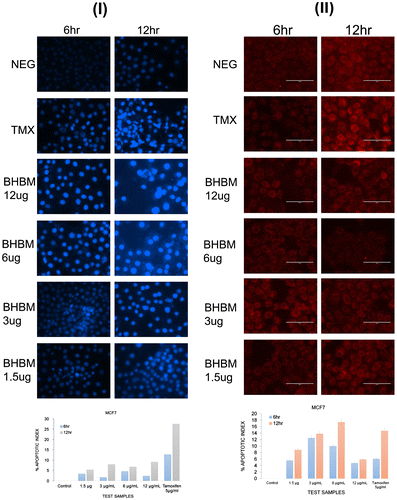
Figure 8. Visualization of Ligand and protein interaction profile: surface visualization (I-A) and cartoon display (I-B) of NF-κB and active site residue interaction of protein NF-κB (I-D, I-F, I-H).
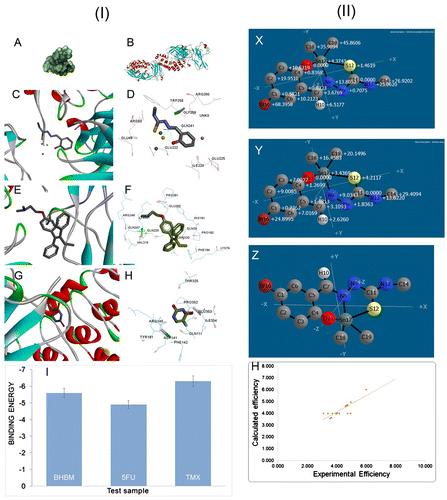
Figure 9. Anticancer activity of BHBM through the induction of apoptotic cell death.
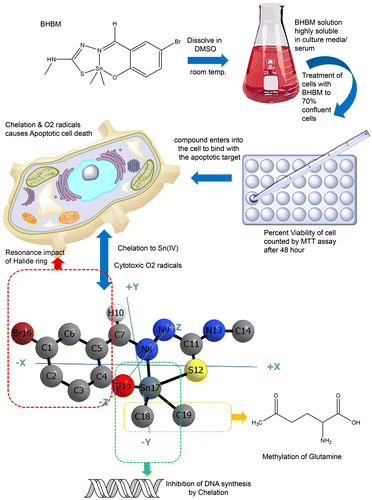
Scheme 1. Synthesis of dimethyltin(IV)-5-bromo-2-hydroxybenzaldehyde-N(4)-methylthiosemicarbazide complex.