Figures & data
Table 1. Purification of phytocystatin from almond
Figure 1. (A) Native-PAGE of total protein (lane 1) and purified almond cystatin (lane 2). (B) Native PAGE of purified almond cystatin. Each lane contains protein obtained from different fraction having maximum inhibitory activity against papain. (C) 2D gel electrophoresis of purified inhibitor with protein molecular weight markers.
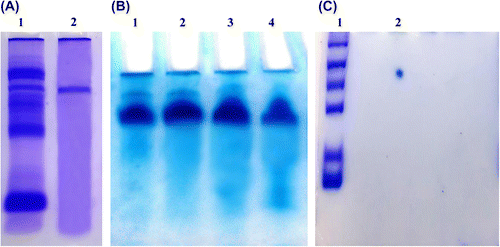
Figure 2. (A) Log M of marker proteins was plotted against relative mobility (Rm) of molecular weight markers for determination of molecular mass of almond cystatin. Molecular weight of standard marker proteins is A, Phosphorylase b (97.4 kDa); B, Bovine Serum Albumin (66 kDa); C, Ovalbumin (43 kDa); D, Carbonic Anhydrase (29 kDa); E, Soyabean Trypsin Inhibitor (20.1 kDa); F, Lysozyme (14.3 kDa). (B) Molecular weight determination of purified almond cystatin using Sephacryl S-100 HR gel filtration chromatography. Purified almond cystatin was applied on a column of Sephacryl S-100 HR (60 × 1.7 cm) and eluted with 50 mM sodium phosphate buffer, pH 7.5 at a flow rate of 15 ml h−1. The molecular weight markers used were, cytochrome c (12 kDa), lysozyme (14.3 kDa) soyabean trypsin inhibitor (20 kDa), ovalbumin (45 kDa), BSA (68 kDa), and phosphorylase b (97.4 kDa). Arrow shows the position of almond cystatin elution.
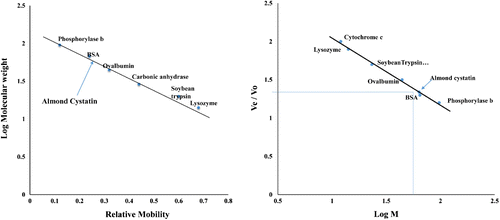
Figure 3. Inhibitory activity of almond cystatin for different proteases.
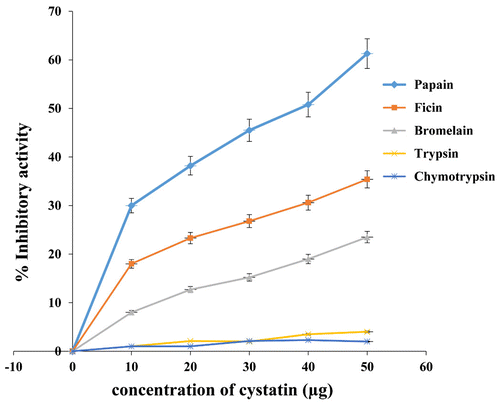
Figure 4. (A) Stoichiometric titration of papain with almond cystatin. Residual activity of papain was determined using casein as a substrate. (B) Effect of temperature on inhibitory activity of almond cystatin. 50 μg of cystatin in 50 mM sodium phosphate buffer, pH 7.5, was incubated at various temperatures for 60 min. Remaining % inhibitory activity was analyzed against 50 μg of papain. (C) Effect of pH on inhibitory activity of almond cystatin. 50 μg of cystatin was incubated in 50 mM sodium acetate buffer pH 3–6, sodium phosphate buffer, pH 7–8 and Tris-HCl buffer pH 9–12 at 37°C. Remaining % inhibitory activity was analysed against 50 μg of papain 37°C.
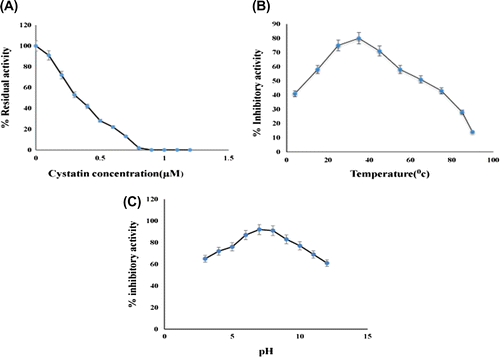
Figure 5. (A) Ouchterlony immunodiffusion: Antibodies carrying antiserum against almond cystatin was raised in rabbits. For the immunodiffusion study, the antiserum is allowed to react with cystatin on agarose plates. The central well contained the antiserum, whereas the surrounding three wells contained almond cystatin (A) 30 μg, (B) 40 μg, and (C) 50 μg. (B)Western immunoblot of the purified almond cystatin showed prominent antigenic bands. Lane a, b, c, d, e, f shows different concentrations of purified cystatin used.
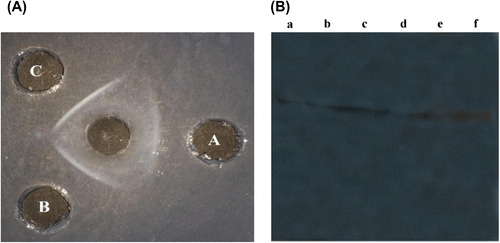
Figure 6. Determination of inhibition constant Ki with papain. Papain was used at a final concentration of 0.06 nM with increasing amount of inhibitor (0.06–0.30 nM) and measuring the residual activity using casein as substrate.
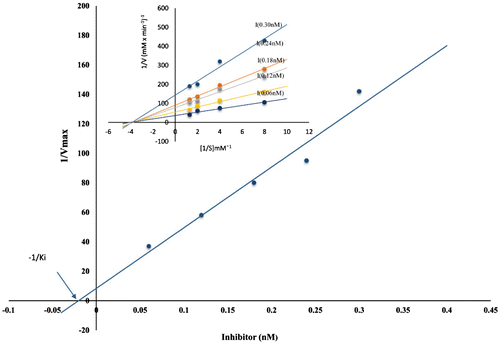
Table 2. Kinetic constants obtained on interaction of almond cystatin with proteases—papain, ficin, and bromelain
Figure 7. (A) Ultraviolet absorption spectra for almond cystatin bound to papain. For complex formation with papain, almond cystatin concentration used was 40 μg/1.5 ml. (B) Fluorescence spectra of cystatin in complex with papain. A fluorescence spectrum of almond cystatin, papain, and papain–cystatin complex was measured at excitation wavelength 280 nm and emission wavelength 300–400 nm. The concentration of almond cystatin was 40 μg/1.5 ml. (C) Far-UV CD spectrum of almond cystatin 20 μm was recorded between 200 and 250 nm using 10-mm path length. (D) FTIR spectra of cystatin alone (2 mM) prepared in sodium phosphate buffer (50 mM, pH 7.5), and cystatin with papain (0.5 mM) was prepared in sodium phosphate buffer (50 mM, pH 7.5). Average of five scan was taken. The spectrum of bound form cystatin was obtained by subtraction of the spectrum of papain from the cystatin + papain complex.
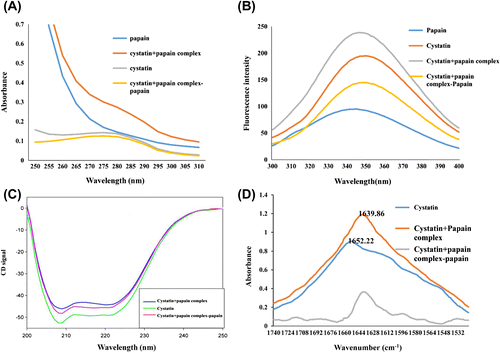
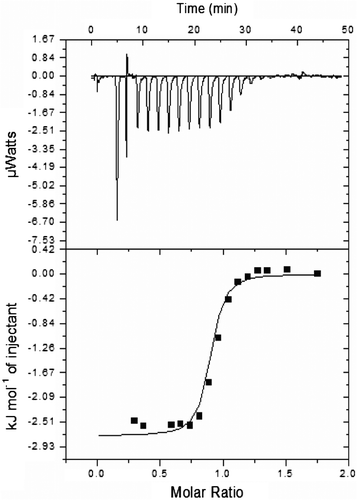
Figure 8. Isothermal titration calorimetry of almond cystatin titrated with papain.