Figures & data
Figure 1. Structures of salient species in the assessment of the nonenzymatic covalent protein modification of HbA by acetaldehyde or glyceraldehyde, with potential effector reagents 2,3-bisphosphoglycerate (2,3-BPG) and inorganic phosphate (Pi).
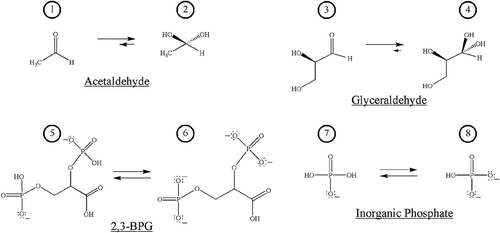
Figure 2. Generic scheme for the nonenzymatic covalent protein modification (NECPM) of a protein by a non-glucose binding species.

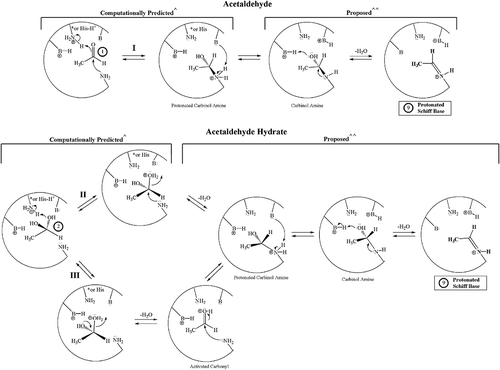
Figure 3. Potential NECPM mechanisms for covalent modification of Val1 of HbA that are geometrically possible for acetaldehyde 1 (Mechanism I) and the acetaldehyde hydrate 2 (Mechanisms II and III) based upon molecular modeling with MOE.1
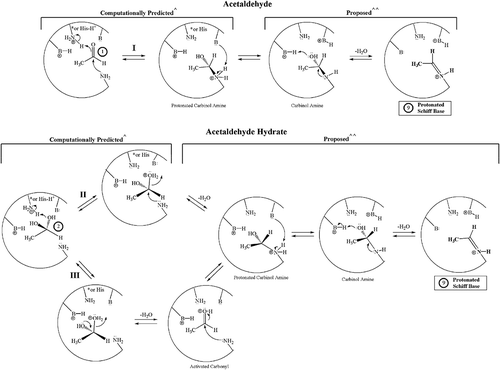
Table 1. Computational modeling of acetaldehyde (aldehyde 1 and hydrate 2) binding in the HbA1c pocket of human hemoglobin as both single-species binding and concomitant binding with potential effector reagents, 2,3-BPG (5 or 6) or Pi (7 or 8)
Table 2. Computational modeling of Glyceraldehyde/Hydrate binding to the HbA1c pocket of human hemoglobin both as single-species binding and with concomitant binding with 2,3-BPG or with Pi
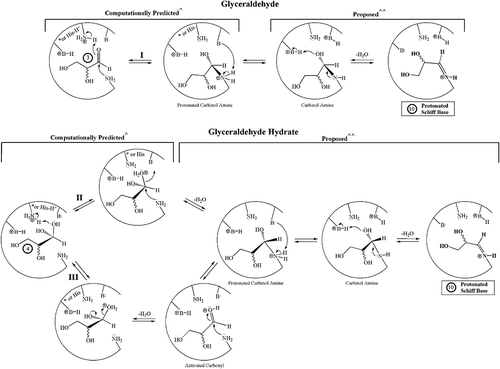
Figure 4. Potential NECPM mechanisms for HbA covalent modification that are geometrically possible for glyceraldehyde 3 (Mechanism I) and the glyceraldehyde hydrate 4 (Mechanisms II and III) based upon molecular modeling with MOE.2