Figures & data
Figure 1. Schematic model for neuronal pathways controlling cutaneous vasomotor activity. Cutaneous vasoconstrictor sympathetic premotor neurons in the medullary raphé excite cutaneous vasoconstrictor sympathetic preganglionic neurons in the spinal intermediolateral nucleus at least in part by serotonergic (5-HT) activation of 5-HT2A receptors and by glutamatergic (GLU) activation. Excitatory drive from the rostral ventrolateral medulla (RVLM) contributes to maintaining cutaneous sympathetic tone. Temperature-responsive neurons in the caudolateral preoptic region (CLPO) and rostromedial preoptic region (RMPO) provide thermoregulatory control of cutaneous vasomotor response to cold or warm stimuli. Warm-responsive preoptic neurons send direct inhibitory (GABAergic) projections to the medullary raphé. The warm-responsive neurons inhibit cutaneous sympathetic premotor neurons under warm-condition and contribute to cutaneous vasodilator response. Some of warm-responsive neurons exert inhibitory influence on cutaneous sympathetic outflow by inhibiting cutaneous vasoconstrictor neurons in the ventral tegmental area (VTA) that may provide excitatory drive to the medullary raphé neurons, or by activating cutaneous vasodilatative neurons in the rostral ventrolateral periaqueductal gray (rvlPAG) that may provide inhibitory drive the medullary raphé neurons. Cold-responsive RMPO neurons send direct excitatory (glutamatergic) projections to the medullary raphé. The cold-responsive neurons excite cutaneous sympathetic premotor neurons in the cold, and contribute to cutaneous vasoconstriction. The cold-responsive RMPO neurons receive tonic GABAergic inputs under warm-condition. PGE2 possibly inhibits GABAergic interneurons in the RMPO that send direct projection to cold-responsive neurons in the RMPO, and elicit cutaneous vasoconstriction by reducing inhibitory influence on the medullary raphé neurons. The GABAergic interneurons may also exert inhibitory influence on the medullary raphé neurons via other indirect pathway. During aversive psychological events, cutaneous vasoconstrictor sympathetic premotor neurons in the medullary raphé are activated at least in part by excitatory drive from neurons in the amygdala, the dorsomedial hypothalamus (DMH), and orexinergic neurons in the hypothalamic area (lateral hypothalamus (LH), perifornical area (PeF) and DMH). Noradrenergic neurons in the locus coeruleus (LC) contribute to the excitatory drive via the amygdala. Activation of the neurons in the habenula elicits cutaneous vasoconstriction possibly by activation of the medullary raphé neurons. Solid black line with a question mark indicates that the pathway is not established. Solid black with dashed lines indicates that it is not known whether pathway is direct or indirect.
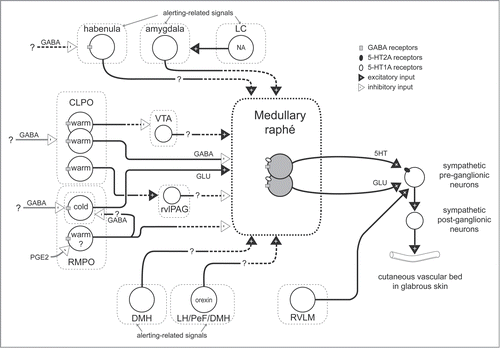
Figure 2. Cutaneous sympathetic nerve is activated by truncal skin cooling in anesthetized rabbits (A) and rats (B). These responses are abolished after microinjection of muscimol (1 nmol in 100 nl) or glycine (100 nmol in 200 nl) into the medullary raphé. The circled numbers (1-3) in each graph correspond to the circled numbers on the bottom X-axis, indicating the experimental period during which the nerve recording was made. Insets show transverse sections of rostral medulla oblongata showing injection sites in rabbit (A) and in rat (B). MVe, medial vestibular nucleus; py, pyramidal tract; VII, facial nucleus. Modified from Ootsuka et al.Citation22 © Elsevier. Permission to reuse must be obtained from the rightsholder.
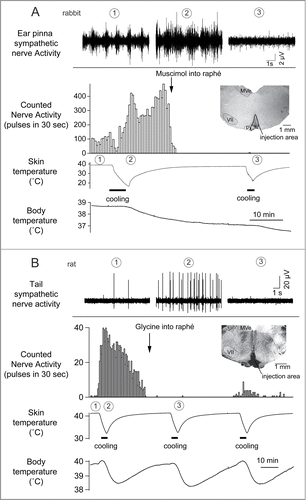
Figure 3. The increase in tail skin temperature elicited by warming the preoptic area (POA) is suppressed by blocking inhibitory input to neurons in the medullary raphé, not in the rostral ventrolateral medulla (RVLM) with bicuculline (150 pmol, in 300 nl per site, arrows) in anesthetized rats. Modified from Tanaka et al.Citation23 © Wiley. Permission to reuse must be obtained from the rightsholder.
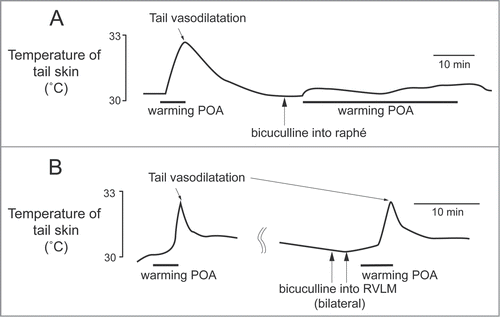
Figure 4. Tail sympathetic fiber response to bilateral inhibition of RVLM neurons with muscimol (336 pmol in 160 nl per side, 2 arrows) or to inhibition of the medullary raphé neurons with muscimol (360 pmol in 120nl) in anesthetized rats. (A) After the inhibition of the RVLM neurons, either of L-glutamate (L-Glu, 6 nmol in 120 nl) injection into the medullary raphé or strong cooling was still able to activate tail sympathetic fibers. (B) After neuronal inhibition in the medullary raphé, either of L-Glu injection into the RVLM or strong cooling fails to reactivate the tail fibers. Horizontal bars on skin temperature traces show periods when cooling was performed. Modified from Ootsuka and McAllen.Citation51 © American Physiological Society. Permission to reuse must be obtained from the rightsholder.
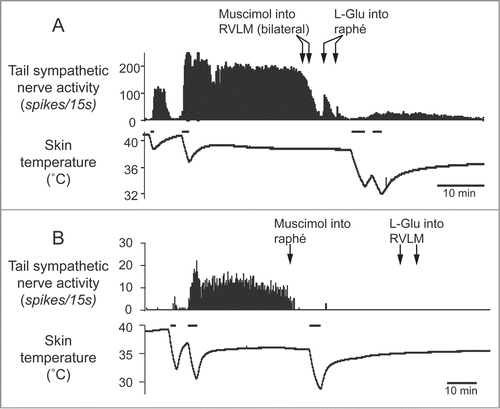
Figure 5. Warming the preoptic area (POA) significantly reduces cold-elicited increase in tail sympathetic nerve activity in anesthetized rats. The left record shows a control cutaneous sympathetic excitatory response to cooling via the water jacket. The right record shows the cold-elicited response was reversed by preoptic warming to 45°C. The inset shows thermode tip locations in 4 experiments (black dots) on a coronal section of the preoptic area. An arrow marks the site warmed in the record. ac, anterior commissure nucleus; CPu, caudate-putamen; LV, lateral ventricle; OX, optic chiasm. Modified from Owens et al.Citation59 © Wiley. Permission to reuse must be obtained from the rightsholder.
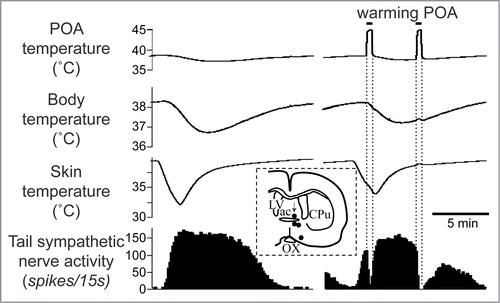
Figure 6. Thermo-responsive raphé-projecting neurons in the preoptic area in anesthetized rats. (A) Cold-responsive that are activated by skin cooling and inhibited by skin warming. (B) Warm-responsive neurons that are activated by skin warming and inhibited by skin cooling. (C, D) Drawing of rostral and caudal coronal sections in the preoptic area showing some of identified cold-responsive (gray circle) and warm-responsive (white circle) neurons that are antidromically activated by electrical stimulation in the medullary raphé. (E) Distribution of all identified warm- /cold-responsive raphé-projecting neurons in the horizontal plane reconstructed from sequential coronal planes. Crosses show thermo-insensitive raphé-projecting neurons. Shaded areas show the RMPO and CLPO.Citation30 3V, Third ventricle; ac, anterior commissure; AVPV, anteroventral periventricular nucleus; f, fornix; HDB, horizontal limb of the diagonal band of Broca; MnPO, median preoptic area; MPN, medial preoptic nucleus; MPO, medial preoptic area; LPO, lateral preoptic area; LV, lateral ventricle; OVLT, organum vasculosum of the lamina terminalis; ox, optic chiasm. Modified from Tanaka et al.Citation30 © Society for Neuroscience. Permission to reuse must be obtained from the rightsholder.
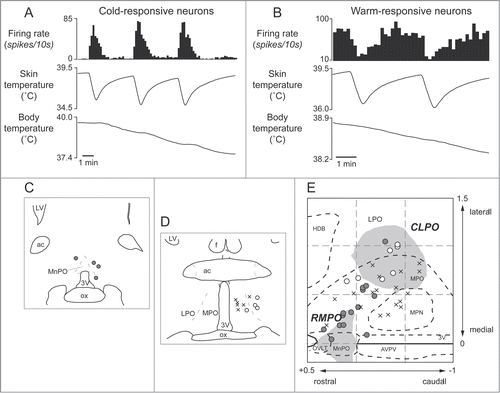
Figure 7. Microinjection of bicuculline (7.5 pmol in 15 nl) into the RMPO (A) but not into the CLPO (B) activate tail sympathetic fibers in anesthetized rats. Modified from Tanaka et al.Citation30 © Society for Neuroscience. Permission to reuse must be obtained from the rightsholder.
Figure 8. Blocking excitatory inputs to the medullary raphé with kynurenate (6 nmol in 120 nl) inhibits tail sympathetic excitatory response to skin cooling. Modified from Tanaka et al.Citation30 © Society for Neuroscience. Permission to reuse must be obtained from the rightsholder.
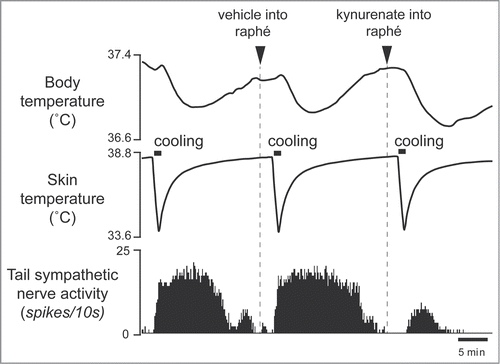
Figure 9. Blocking excitatory inputs to the medullary raphé with kynurenate inhibits tail sympathetic nerve activity elicited by microinjection of bicuculline (7.5 pmol in 15 nl) into the RMPO in anesthetized rats. Vehicle (artificial cerebrospinal fluid, 120nl) (A) or kynurenate (6 nmol in 120 nl) (B) was injected into the medullary raphé. Modified from Tanaka et al.Citation30 © Society for Neuroscience. Permission to reuse must be obtained from the rightsholder.
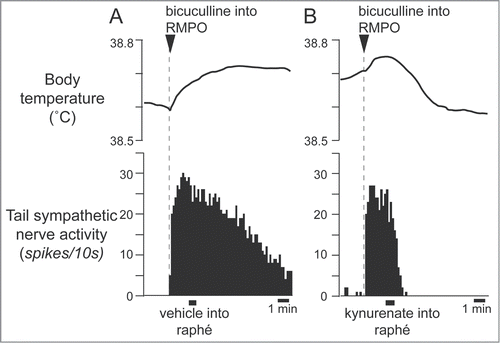
Figure 10. Blocking excitatory inputs to the medullary raphé with kynurenate inhibits tail sympathetic excitatory response to PGE2 (0.2 ng in 60 nl) injected into the RMPO in anesthetized rats. Vehicle (artificial cerebrospinal fluid, 120nl) (A) or kynurenate (6nmol in 120 nl) (B) was injected into the medullary raphé. Modified from Tanaka et al.Citation34 © American Physiological Society. Permission to reuse must be obtained from the rightsholder.
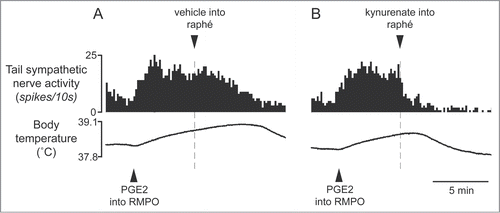
Figure 11. Bilateral injections of bicuculline into the dorsal preoptic area (POA) increases tail skin temperature during fever response elicited by intravenous injection of lipopolysaccharide (LPS) (1 µg/kg) in anesthetized rats. Solid arrows show the times when bicuculline (500 pmol in 100 nl) or saline (100 nl) was microinjected into the dorsal POA at intervals of 40 min. Bicuculline-injection group was shown by filled circles (n = 4 ). Saline-injection group was shown by open circles (n = 4 ). Modified from Osaka.Citation70 © Elsevier. Permission to reuse must be obtained from the rightsholder.
Figure 12. Electrical (0.2 mA, 200 ms, 30 Hz) and pharmacological (D,L-homocysteic acid, 300 pmol in 300 nl ) stimulation in the ventral tegmental area (VTA) reverse increases in tail skin temperature and tail blood flow elicited by warming the preoptic area (POA) in anesthetized rats. A stimulation site (black dot) in the VTA is shown in the inset. ML, medial lemniscus; MP, mammillary nucleus; PAG, periaqueductalgrey. Modified from Zhang et al.Citation24 © Wiley. Permission to reuse must be obtained from the rightsholder.
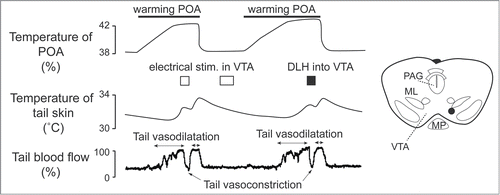
Figure 13. Intravenous administration of 5-hydroxytryptamine (5HT) 1A receptor agonist (8-OH-DPAT) inhibits cold-induced ear pinna cutaneous vasoconstriction in conscious rabbits. Microinjection of 5-HT1A receptor antagonist (WAY-100635), into the medullary raphé/parapyramidal region reverses the ear pinna cutaneous vasomotor response to intravenous administration of 8-OH-DPAT. Records of ultrasonic Doppler signal measuring phasic ear pinna blood flow in conscious freely moving rabbits. Modified from Ootsuka and Blessing.Citation101 © Elsevier. Permission to reuse must be obtained from the rightsholder. The second intravenous injection of 8-OH-DPAT after the WAY-100635 did not cause significant effect on cutaneous blood flow, confirming antagonize action of WAY-100635 on 8-OH-DPAT.
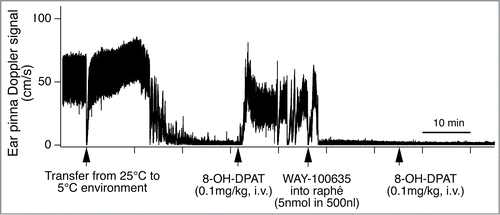
Figure 14. Intravenous administration of 5-hydroxytryptamine (5HT) 2A agonist (±)-1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane (DOI) elicits ear pinna cutaneous vasoconstriction. (A) The 5HT2A agonist DOI (100 µg/kg i.v.) decreases Doppler blood flow signal selectively in ear pinna not in mesenteric artery, and increases body temperature in a conscious rabbit. Modified from Blessing and Seaman.Citation107 © Elsevier. Permission to reuse must be obtained from the rightsholder. (B) Microinjection of muscimol into the medullary raphé (1 nmol in 100 nl) inhibits spontaneous cutaneous sympathetic activity in ear pinna in an anesthetized rabbit. Subsequent DOI (0.1 mg/kg, i.v.) administration activates ear pinna sympathetic fiber. The circled numbers (1-3) on the nerve discharge traces correspond to the circled numbers on the X axis in the integrated nerve activity traces, indicating the experimental period during which the nerve recording was made. Modified Ootsuka et al.Citation106© Elsevier. Permission to reuse must be obtained from the rightsholder.
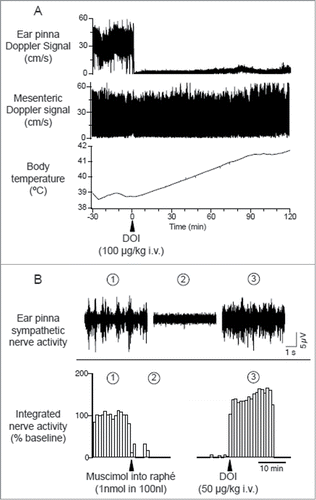
Figure 15. Raphé-elicited ear pinna sympathetic nerve discharge is reduced by 5-hydroxytryptamine (5HT) 2A receptor antagonist, SR-46349B to an isolated cerebrospinal fluid pool (CSF), between T1-T7 thoracic spinal segments in anesthetized rabbits. The remaining response is substantially reduced by subsequent glutamate receptor antagonist, kynurenate. Peri-stimulus average (16 sweeps) of ear pinna sympathetic nerve discharge evoked by triple-pulse electrical stimulation (25 µA, 0.5 ms, 3 pulses at 100 Hz) of the medullary raphé after application to the isolated CSF pool of vehicle (A), SR-46349B (80 µg/kg, 0.8 ml; B), and kynurenate (25 µmol in 0.5 ml; C). Modified from Ootsuka and Blessing.Citation108 © American Physiological Society. Permission to reuse must be obtained from the rightsholder.
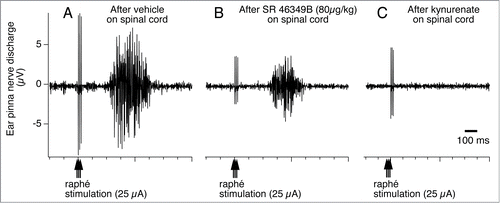
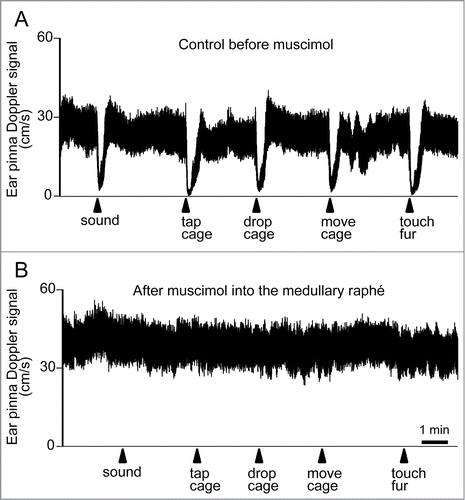
Figure 16. Ear pinna Doppler blood flow signal demonstrating cutaneous vasoconstriction elicited by salient stimuli before (A) and after (B) muscimol (3 nmol in 300 nl) injection into the medullary raphé in conscious rabbits. Salient stimuli (a brief sound, cage tap, sudden 1 cm drop of cage, sideways movement of the cage and touching of the animals fur. See details in refsCitation129,139) were applied at the time point indicated by arrows. Modified from Ootsuka and Blessing.Citation20 © Elsevier. Permission to reuse must be obtained from the rightsholder.