Figures & data
Figure 1. Sleep bioassay system for rodents. (A) To monitor electroencephalogram (EEG) signals, stainless steel screws are implanted epidurally over the frontal cortical and parietal areas of one hemisphere. In addition, electromyogram (EMG) activity is monitored by stainless steel, teflon-coated wires placed bilaterally within the trapezius muscles. (B) wakefulness (i) is characterized by low to moderate voltage EEG and the occurrence of EMG activity, whereas NREM sleep (ii) is identified by the appearance of large, slow brain waves with a rhythm below 0.5–4 Hz (orange frequencies in the fast Fourier transform, FFT, of the EEG) and REM sleep (iii), exhibits a shift back to a rapid low-voltage EEG and the appearance of brain waves in the theta range, i.e., 6–10 Hz (blue frequencies in FFT of the EEG).
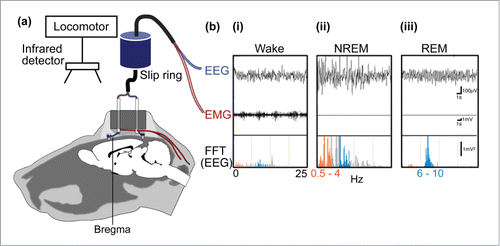
Figure 2. Circuit basis of sleep-wake regulation. Model 1 (shown in panel a): Adenosine inhibits the release of acetycholine from basal forebrain (BF) cholinergic neurons to produce slow-wave sleep. Model 2 (shown in panels b-d): a flip–flop switching mechanism involving mutually inhibitory interactions between sleep-promoting neurons in the ventrolateral preoptic area (VLPO) and wake-promoting neurons in the hypothalamus [i.e., histaminergic tuberomammilary nucleus (TMN)], and brainstem [i.e., noradrenergic locus coeruleus (LC), serotonergic dorsal raphe nucleus (DR), and cholinergic laterodorsal tegmental nucleus (LDT)]. The flip-flop switch between the VLPO and hypothalamus and brainstem is stabilized by orexin/hypocretin (OX/Hcrt) inputs. Adenosine is known to act as an endogenous somnogen and promotes sleep via inhibitory A1 receptors (A1) in the basal forebrain, VLPO and TMN and excitatory A2A receptors (A2A) in the nucleus accumbens (NAc) and VLPO.Citation98-101 Other Abbreviations: Ach, acetylcholine; 5-HT, serotonin, NE, norepinephrine.
![Figure 2. Circuit basis of sleep-wake regulation. Model 1 (shown in panel a): Adenosine inhibits the release of acetycholine from basal forebrain (BF) cholinergic neurons to produce slow-wave sleep. Model 2 (shown in panels b-d): a flip–flop switching mechanism involving mutually inhibitory interactions between sleep-promoting neurons in the ventrolateral preoptic area (VLPO) and wake-promoting neurons in the hypothalamus [i.e., histaminergic tuberomammilary nucleus (TMN)], and brainstem [i.e., noradrenergic locus coeruleus (LC), serotonergic dorsal raphe nucleus (DR), and cholinergic laterodorsal tegmental nucleus (LDT)]. The flip-flop switch between the VLPO and hypothalamus and brainstem is stabilized by orexin/hypocretin (OX/Hcrt) inputs. Adenosine is known to act as an endogenous somnogen and promotes sleep via inhibitory A1 receptors (A1) in the basal forebrain, VLPO and TMN and excitatory A2A receptors (A2A) in the nucleus accumbens (NAc) and VLPO.Citation98-101 Other Abbreviations: Ach, acetylcholine; 5-HT, serotonin, NE, norepinephrine.](/cms/asset/838c2a90-95f1-40fb-a23c-d7e55b412193/ktmp_a_1075095_f0002_c.gif)
Figure 3. Cre-lox system. (A) The 34-base pair loxP sequence consists of an 8-base pair core sequence where recombination takes place (white box) and 2 flanking 13-base pair inverted repeats. (B–D) The outcome of recombination by Cre, a P1 Bacteriophage enzyme that is encoded by the locus originally named as “causes recombination”, is determined by the location and orientation of lox sites. (B) Cre mediates the inversion of the floxed DNA segment, if lox sites are oriented in opposite directions. (C) Cre mediates a deletion of the floxed DNA segment, if lox sites are oriented in the same direction. (D) Cre mediates a translocation, if lox sites are located on different chromosomes.
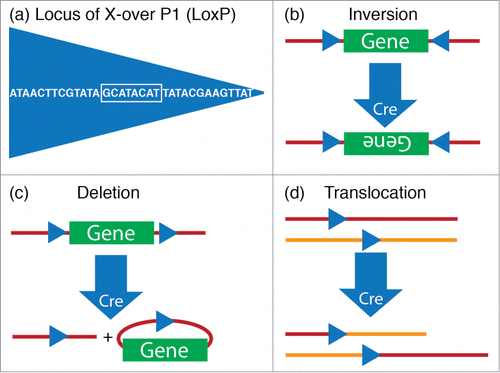
Figure 4. The arousal effects of caffeine are abolished in rats with site-specific deletion of A2A receptors (A2AR) in the shell of the nucleus accumbens (NAc). To identify the neurons on which caffeine acts to produce arousal, A2A receptors were focally depleted by bilateral injections of adeno-associated virus carrying short-hairpin RNA for the A2A receptor into the core (dashed green line in the left panel) or shell (dashed red line in the right panel) of the NAc of rats.Citation61 Typical hypnograms that show changes in wakefulness and in rapid eye movement (REM) and non-REM (NREM) sleep after administration of caffeine at a dose of 15 mg/kg indicate that rats with a shell, but not a core, knockdown of the A2A receptors showed a strongly attenuated caffeine arousal. Green and red areas in the hypnograms represent wakefulness after caffeine administration that correspond to the depletion of A2A receptors in the respective core and shell of the NAc.
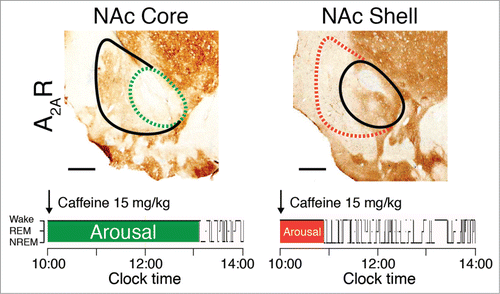
Figure 5. Photostimulation of neurons in behaving animals: combining Cre-driver mice and stereotaxic-based delivery of adeno-associated virus (AAV) expressing Cre-dependent channelrhodopsin (ChR2). ChR2 are nonspecific cation channels, conducting H+, Na+, K+, and Ca2+ ions. ChR2 absorbs blue light with an absorption spectrum maximum at 480 nm resulting in the opening of a pore in the trans-membrane protein and depolarization of neurons by allowing for the flow of ions according to their electrochemical gradient.Citation102,103 Other Abbreviations: Cre, Cre recombinase; EF1α, archaeal elongation factor 1 α; loxP, locus of X-over P1; lox2272, variant of loxP; pA, poly A tail; WRPE, woodchuck hepatitis virus posttranscriptional regulatory element.
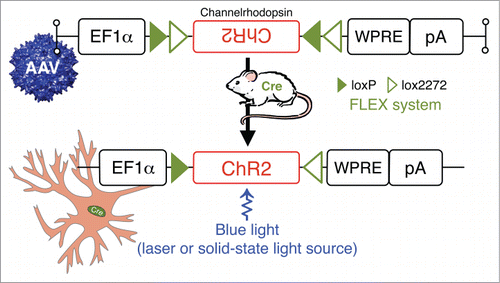
Figure 6. Inducible gene expression by using tetracycline-controlled transcriptional activation (Tet expression systems). Gene transcription is reversibly turned on or off in the presence of the antibiotic tetracycline (Tc) or doxycycline (DOX), a more stable tetracycline analog. In a Tet-Off system, tetracycline and its derivatives bind transactivator protein (tTA) and render it incapable of binding to the tetracycline response element (TRE) consisting of several TetO sequences and a minimal promoter, thereby preventing transcription of TRE-controlled genes. A Tet-On system works similarly, but the rtTA protein is capable of binding to the TRE operator, and hence initiating transcription of the transgene, only when bound by DOX. For most purposes, there is no inherent advantage of using the Tet-Off system over the Tet-On system, although there is no apparent literature example in which the Tet-Off system has been used to study the regulation and function of sleep.
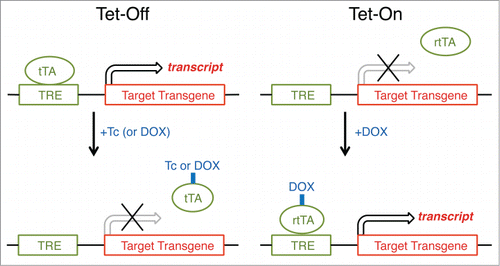
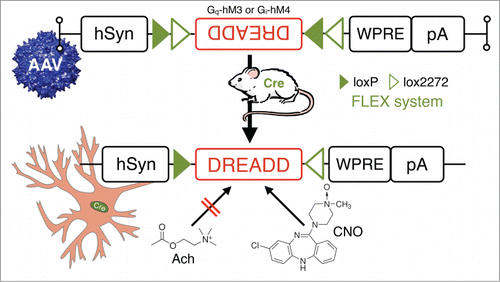
Figure 7. In vivo chemogenetic inhibition or activation of neurons in behaving animals: combining Cre-driver mice and stereotaxic-based delivery of adeno-associated virus (AAV) expressing Cre-dependent “designer receptor exclusively activated by designer drugs” (DREADD). DREADD permit temporal control of excitatory or inhibitory G-protein coupled receptor signaling in vivo by utilizing mutated human muscarinic acetylcholine (AcH) receptors.Citation82,83 These AcH receptors, excitatory hM3 and inhibitory hM4, are unresponsive to their natural ligand acetylcholine, but can be activated by nanomolar doses of the synthetic small-molecule clozapine-N-oxide (CNO). Other Abbreviations: Cre, Cre recombinase; hsyn, human synapsin promoter; loxP, locus of X-over P1; lox2272, variant of loxP; pA, poly A tail; WRPE, woodchuck hepatitis virus posttranscriptional regulatory element.