Figures & data
Figure 1. Cutaneous nerve block eliminates the increase in forearm blood flow during heat stress. One of the first studies to provide evidence that the increase in forearm blood flow during heat stress was due to a reflex vasodilation rather than withdrawal of vasoconstriction. Forearm blood flow with cutaneous nerve block is shown in the filled symbols and forearm blood flow to the control limb is shown in open symbols. Note the near elimination of forearm vasodilation during heating in the nerve-blocked limb. Adapted, with permission, from Edholm et al.Citation7
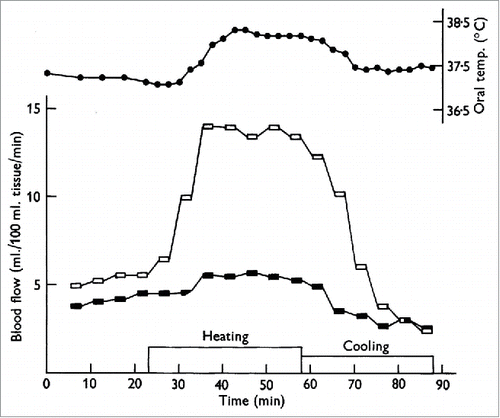
Figure 2. Increase in forearm blood flow is due largely to an increase in skin, rather than skeletal muscle, blood flow. Iontophoresis of adrenaline completely arrests the skin circulation but has little to no effect on skeletal muscle circulation. There was little to no increase in forearm blood flow during heat stress following iontophoresis of adrenaline to the right forearm (filled symbols) compared to the substantial increase in forearm blood flow to the control limb (open symbols). Adapted, with permission, from Edholm et al.Citation13
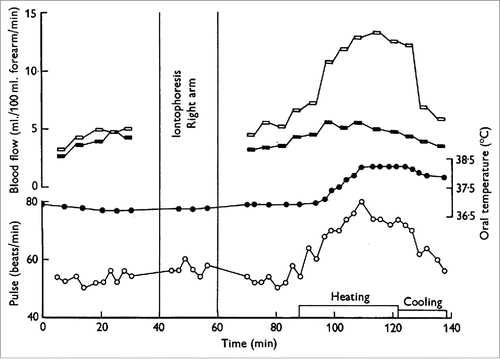
Figure 3. NO component of cutaneous active vasodilation is mediated by nNOS. Inhibition of nNOS with 7-NI (filled symbols) attenuates cutaneous active vasodilation, suggesting nNOS is responsible for the NO component of active vasodilation. NOS inhibition had no effect on skin blood flow during cold stress (CS). Adapted, with permission, from Kellogg et al.Citation45
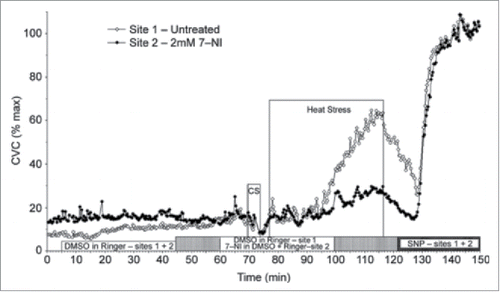
Figure 4. Contribution of NO to cutaneous vasodilation during dynamic exercise. Similar to passive heat stress, NO contributes ∼35% to cutaneous vasodilation during dynamic exercise sufficient to increase in core temperature ∼0.8°C above baseline. Whereas NO appears to be derived via nNOS during passive heat stress, the NO component during dynamic exercise appears to be derived from eNOS. Adapted, with permission, from McNamara et al.Citation46
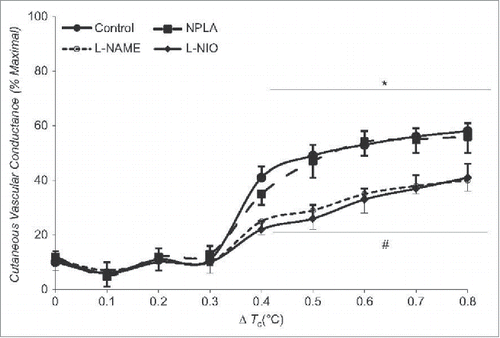
Figure 5. Effect of arginase inhibition and L-Arginine supplementation on cutaneous active vasodilation in young and older humans. Cutaneous active vasodilation is attenuated in older (open bars) compared to younger (black bars) subjects. Inhibition of arginase, L-Arginine supplementation, or a combination of the 2 restored cutaneous active vasodilation in older subjects but had no effect in younger subjects. These data suggest that healthy aging results in an increase in arginase activity that reduces bioavailability of L-Arginine, an important substrate for NOS. Adapted, with permission, from Holowatz et al.Citation109
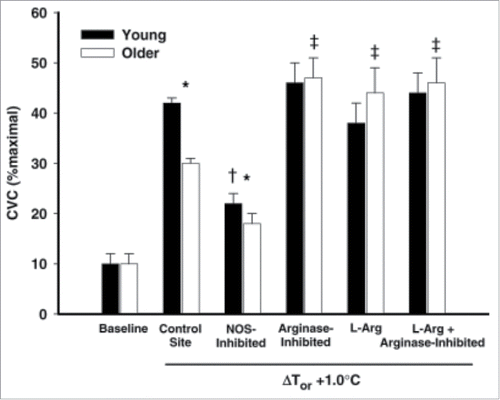
Figure 6. Attenuated NO-dependent cutaneous active vasodilation in patients with essential hypertension. Patients with essential hypertension have a reduced NO component to cutaneous active vasodilation during heat stress at core temperatures >0.5°C. ΔCVC was calculated as the difference between control and NOS inhibited sites to obtain an index of the NO-contribution for each 0.1°C increase in core temperature. Adapted, with permission, from Holowatz et al.Citation129
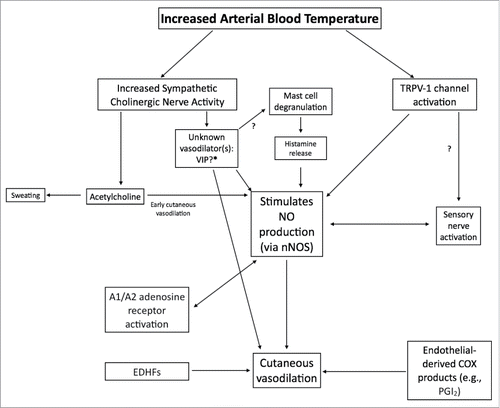
Figure 7. Schematic flowchart of vasodilators and pathways involved in cutaneous active vasodilation. Although most of the identified vasodilators are depicted, for clarity, not all receptors and downstream second messenger pathways are shown. Arrows marked with a “?” indicate pathways that are speculative and lack empirical data.