Figures & data
Figure 1. Mean (±SEM) changes in brain (ventral striatum, dorsal striatum, and cerebellum) and arterial blood temperatures induced by environmental change (animal transfer from the vivarium to the recording chamber), social interaction (3-min placement of a female rat into the chamber with recorded male), tail-pinch (3-min placement of wooden clamp on the tail base), and unexpected auditory stimulus (20-s tone) in freely moving male rats. A = absolute temperatures; B = relative temperature changes; C = brain-arterial blood temperature differences. Filled symbols indicate values significantly higher (Scheffe’s test following one-way ANOVA with repeated measures, p < 0.05) than baseline. Duration of stimuli is shown by two vertical hatched lines. The minimal value within each session, when the animal was at rest, was taken as baseline for the environmental change. Data were replotted from [Citation36].
![Figure 1. Mean (±SEM) changes in brain (ventral striatum, dorsal striatum, and cerebellum) and arterial blood temperatures induced by environmental change (animal transfer from the vivarium to the recording chamber), social interaction (3-min placement of a female rat into the chamber with recorded male), tail-pinch (3-min placement of wooden clamp on the tail base), and unexpected auditory stimulus (20-s tone) in freely moving male rats. A = absolute temperatures; B = relative temperature changes; C = brain-arterial blood temperature differences. Filled symbols indicate values significantly higher (Scheffe’s test following one-way ANOVA with repeated measures, p < 0.05) than baseline. Duration of stimuli is shown by two vertical hatched lines. The minimal value within each session, when the animal was at rest, was taken as baseline for the environmental change. Data were replotted from [Citation36].](/cms/asset/a3950a75-8e56-4c30-869a-869a5577b8e7/ktmp_a_1691896_f0001_oc.jpg)
Figure 2. Onset latencies of temperature responses to different environmental challenges in brain structures and arterial blood in freely moving rats. Mean values are shown at 2-s intervals for 10 s before and 60 s after the onset of each stimulus. Filled symbols indicate values significantly (one-way ANOVA followed by Scheffe test, p < 0.05) higher than the last pre-stimulus value. Response latencies were defined as the first significant value vs. baseline and shown in brackets. Data were replotted from reference [Citation36].
![Figure 2. Onset latencies of temperature responses to different environmental challenges in brain structures and arterial blood in freely moving rats. Mean values are shown at 2-s intervals for 10 s before and 60 s after the onset of each stimulus. Filled symbols indicate values significantly (one-way ANOVA followed by Scheffe test, p < 0.05) higher than the last pre-stimulus value. Response latencies were defined as the first significant value vs. baseline and shown in brackets. Data were replotted from reference [Citation36].](/cms/asset/e33b49fa-1512-46ed-8dbc-dfedb80c5f96/ktmp_a_1691896_f0002_oc.jpg)
Figure 3. Day-to-day changes in NAc temperature increases induced by different environmental stimuli (tail-pinch, male-male interaction, male-female interaction, auditory stimulus). A = mean (±SEM) increases from baseline for each challenge condition on each day, B = mean (±SEM) duration of statistically significant increase evoked by each challenge on each day. C = mean (±SEM) values of maximal temperature increases during environmental change and mean values of minimal temperature (baseline) on each day. Asterisks mark values significantly different vs. Day 1 (Student’s t-test, p < 0.05). Data were replotted from reference [Citation36].
![Figure 3. Day-to-day changes in NAc temperature increases induced by different environmental stimuli (tail-pinch, male-male interaction, male-female interaction, auditory stimulus). A = mean (±SEM) increases from baseline for each challenge condition on each day, B = mean (±SEM) duration of statistically significant increase evoked by each challenge on each day. C = mean (±SEM) values of maximal temperature increases during environmental change and mean values of minimal temperature (baseline) on each day. Asterisks mark values significantly different vs. Day 1 (Student’s t-test, p < 0.05). Data were replotted from reference [Citation36].](/cms/asset/e4c80d03-d797-4770-a8a2-d2884c2d674c/ktmp_a_1691896_f0003_oc.jpg)
Figure 4. Changes in brain (nucleus accumbens or NAc), temporal muscle, and skin temperatures during tail-pinch, social interaction, and subcutaneous saline injection. A = relative temperature changes; B = brain-muscle and skin-muscle temperature differentials; C = locomotor activity. Duration of stimuli is shown by vertical hatched lines. Filled symbols indicate values significantly (one-way ANOVA followed by Fisher test, p < 0.05) higher than the last pre-stimulus value. Compiled from original data published in [Citation57].
![Figure 4. Changes in brain (nucleus accumbens or NAc), temporal muscle, and skin temperatures during tail-pinch, social interaction, and subcutaneous saline injection. A = relative temperature changes; B = brain-muscle and skin-muscle temperature differentials; C = locomotor activity. Duration of stimuli is shown by vertical hatched lines. Filled symbols indicate values significantly (one-way ANOVA followed by Fisher test, p < 0.05) higher than the last pre-stimulus value. Compiled from original data published in [Citation57].](/cms/asset/cada215e-98c3-44ba-bba8-560f774785d4/ktmp_a_1691896_f0004_oc.jpg)
Figure 5. Relationships between changes in NAc temperature and two temperature differentials (NAc-muscle and skin-muscle) during tail-pinch, social interaction and subcutaneous saline injection in freely moving rats. Graphs on the left side show time-dependent correlative relationships between changes in NAc temperature and two temperature differentials. In each case, increases in NAc temperature correlated with increases in NAc-muscle differentials and decreases in skin-muscle differentials. Graphs on the right side show that NAc temperature increases induced by each arousing stimulus were dependent on basal NAc temperatures; these responses were strong at low basal temperatures and progressively weaker at higher basal temperatures. Regression line crossed the line of no effect at ~39.0–39.5°C, suggesting upper limits for physiological brain temperature increase. Compiled from original data published in [Citation57] and re-analyzed for this report.
![Figure 5. Relationships between changes in NAc temperature and two temperature differentials (NAc-muscle and skin-muscle) during tail-pinch, social interaction and subcutaneous saline injection in freely moving rats. Graphs on the left side show time-dependent correlative relationships between changes in NAc temperature and two temperature differentials. In each case, increases in NAc temperature correlated with increases in NAc-muscle differentials and decreases in skin-muscle differentials. Graphs on the right side show that NAc temperature increases induced by each arousing stimulus were dependent on basal NAc temperatures; these responses were strong at low basal temperatures and progressively weaker at higher basal temperatures. Regression line crossed the line of no effect at ~39.0–39.5°C, suggesting upper limits for physiological brain temperature increase. Compiled from original data published in [Citation57] and re-analyzed for this report.](/cms/asset/05d31836-94e6-44aa-9b62-2e8b9b81dfa1/ktmp_a_1691896_f0005_oc.jpg)
Figure 6. Temperature changes in three brain structures (medial-preoptic hypothalamus or mPOA, nucleus accumbens or NAc, and hippocampus or Hippo) and temporal muscle during sexual interaction session in male and female rats. Left panel shows original records of temperature fluctuations in each recording area in representative male and female rat. A1 and A2 are arousal I and II, respectively. Vertical lines show mounts and intromissions, black triangles with numbers show moments of ejaculation, “female out” depicts the moment when sexual companion was removed from the cage. Right panel shows mean values of absolute temperatures in each recording location (A), relative temperature changes (B), and brain-muscle temperature differentials (C) during sexual interaction session in male and female rats. Asterisks on B show values significantly different (ANOVA with repeated measures followed by Scheffe F-test, p < 0.05) from the previous value. Original data were published in [Citation59,Citation87] and replotted for this article.
![Figure 6. Temperature changes in three brain structures (medial-preoptic hypothalamus or mPOA, nucleus accumbens or NAc, and hippocampus or Hippo) and temporal muscle during sexual interaction session in male and female rats. Left panel shows original records of temperature fluctuations in each recording area in representative male and female rat. A1 and A2 are arousal I and II, respectively. Vertical lines show mounts and intromissions, black triangles with numbers show moments of ejaculation, “female out” depicts the moment when sexual companion was removed from the cage. Right panel shows mean values of absolute temperatures in each recording location (A), relative temperature changes (B), and brain-muscle temperature differentials (C) during sexual interaction session in male and female rats. Asterisks on B show values significantly different (ANOVA with repeated measures followed by Scheffe F-test, p < 0.05) from the previous value. Original data were published in [Citation59,Citation87] and replotted for this article.](/cms/asset/f83803b3-77e4-4f3b-9a56-a67bdb72b580/ktmp_a_1691896_f0006_b.gif)
Figure 7. Phasic changes in brain (NAc, mPOA, and Hippo) and muscle temperatures in male and female rats associated with sexual arousal preceding copulatory behavior. A and B = changes in relative temperatures and brain-muscle differentials for the 35 min after the start of sexual stimulation (vertical hatched lines show arousal 1, 2, and time of free interaction). Filled symbols show the first value significantly different (ANOVA with repeated measures followed by Scheffe test, p < 0.05) from baseline for each of three comparisons (arousal 1, arousal 2, and free interaction). C shows the initial period of stimulation (10-s bins for 120 s). In this case, filled symbols indicate all values significantly different from baseline. Bottom graph shows correlative relationships between the magnitude of NAc temperature increase induced by sexually arousing stimuli in male and female rats and basal temperatures. In both cases, correlation was highly significant (p < 0.01). Regression lines for both males and females cross the line of no effect at ~38.4°C, suggesting that the arousal-related brain temperature increase has its natural limits and disappears at high activity state. Data were obtained in 32 (seven rats) and 17 sessions (five rats) in males and females, respectively. Original data were published in [Citation59,Citation87] and replotted for this article.
![Figure 7. Phasic changes in brain (NAc, mPOA, and Hippo) and muscle temperatures in male and female rats associated with sexual arousal preceding copulatory behavior. A and B = changes in relative temperatures and brain-muscle differentials for the 35 min after the start of sexual stimulation (vertical hatched lines show arousal 1, 2, and time of free interaction). Filled symbols show the first value significantly different (ANOVA with repeated measures followed by Scheffe test, p < 0.05) from baseline for each of three comparisons (arousal 1, arousal 2, and free interaction). C shows the initial period of stimulation (10-s bins for 120 s). In this case, filled symbols indicate all values significantly different from baseline. Bottom graph shows correlative relationships between the magnitude of NAc temperature increase induced by sexually arousing stimuli in male and female rats and basal temperatures. In both cases, correlation was highly significant (p < 0.01). Regression lines for both males and females cross the line of no effect at ~38.4°C, suggesting that the arousal-related brain temperature increase has its natural limits and disappears at high activity state. Data were obtained in 32 (seven rats) and 17 sessions (five rats) in males and females, respectively. Original data were published in [Citation59,Citation87] and replotted for this article.](/cms/asset/91153798-63f0-4170-9e15-7cfb3c2898bf/ktmp_a_1691896_f0007_b.gif)
Figure 8. Changes in brain (NAc and MPAH) and temporal muscle temperatures in male and female rats associated with the initiation of copulatory behavior (A and B) and ejaculation (C and D). Left-side graphs show changes in temperature and brain-muscle differentials preceding and following the first mount/intromission of a session (= 0°C). Filled symbols show values significantly larger (ANOVA with repeated measuresfollowed by Fisher test, p < 0.05) from the pre-mount baseline. In males, temperature increase and rise of brain-muscle differentials began ~2 min before the mount/intromission, but in females, it occurred after this event. Right-side graphs show mean changes in temperature and brain-muscle differentials preceding and following ejaculation (= 0°C) he first mount/intromission of a session (= 0°C). Filled symbols indicate values significantly different (ANOVA with repeated measures followed by Fisher test) from the last pre-ejaculation value. n = the number of averaged events. Original data were published in [Citation59,Citation87] and replotted for this article.
![Figure 8. Changes in brain (NAc and MPAH) and temporal muscle temperatures in male and female rats associated with the initiation of copulatory behavior (A and B) and ejaculation (C and D). Left-side graphs show changes in temperature and brain-muscle differentials preceding and following the first mount/intromission of a session (= 0°C). Filled symbols show values significantly larger (ANOVA with repeated measuresfollowed by Fisher test, p < 0.05) from the pre-mount baseline. In males, temperature increase and rise of brain-muscle differentials began ~2 min before the mount/intromission, but in females, it occurred after this event. Right-side graphs show mean changes in temperature and brain-muscle differentials preceding and following ejaculation (= 0°C) he first mount/intromission of a session (= 0°C). Filled symbols indicate values significantly different (ANOVA with repeated measures followed by Fisher test) from the last pre-ejaculation value. n = the number of averaged events. Original data were published in [Citation59,Citation87] and replotted for this article.](/cms/asset/8a224c0b-f5aa-4f46-82f0-f719441d69d0/ktmp_a_1691896_f0008_b.gif)
Figure 9. Mean changes in temperatures (NAc, temporal muscle, and skin; top row), temperature differentials (middle row) and locomotion (bottom row) during three feeding experiments in trained rats. In the first experiment (A), the rat was satiated and did not show eating during the presentation of food container (two vertical lines = presentation and removal of food container). In the second experiment, the rat was food-deprived (hungry) and it reached food container, removed food sample, and consumed it. In the third experiment, the rat was food-deprived (hungry) but the food container was closed and the rat was unable to retrieve food from the container. Original data were reported in [Citation39] and replotted for this article.
![Figure 9. Mean changes in temperatures (NAc, temporal muscle, and skin; top row), temperature differentials (middle row) and locomotion (bottom row) during three feeding experiments in trained rats. In the first experiment (A), the rat was satiated and did not show eating during the presentation of food container (two vertical lines = presentation and removal of food container). In the second experiment, the rat was food-deprived (hungry) and it reached food container, removed food sample, and consumed it. In the third experiment, the rat was food-deprived (hungry) but the food container was closed and the rat was unable to retrieve food from the container. Original data were reported in [Citation39] and replotted for this article.](/cms/asset/2743209d-d9a5-4345-b8fb-09f5b6580816/ktmp_a_1691896_f0009_oc.jpg)
Figure 10. Slow (A) and rapid (B) temperature changes in the brain (NAc), temporal muscle, and skin during drinking of Coke solution. First vertical line in each graph shows the moment of presentation of glucose-containing cup, gray area shows the duration of drinking. Data analyzed with slow (1-min) time resolution shows mean changes in temperature and locomotion from the moment of cup presentation. Data analyzed with high temporal resolution (10-s) show changes with respect to three critical events (cup presentation, start of drinking, and end of drinking). Filled symbols show values significantly different from either baseline (A) or each individual event of drinking behavior. Original data were reported in [Citation113] and replotted for this article.
![Figure 10. Slow (A) and rapid (B) temperature changes in the brain (NAc), temporal muscle, and skin during drinking of Coke solution. First vertical line in each graph shows the moment of presentation of glucose-containing cup, gray area shows the duration of drinking. Data analyzed with slow (1-min) time resolution shows mean changes in temperature and locomotion from the moment of cup presentation. Data analyzed with high temporal resolution (10-s) show changes with respect to three critical events (cup presentation, start of drinking, and end of drinking). Filled symbols show values significantly different from either baseline (A) or each individual event of drinking behavior. Original data were reported in [Citation113] and replotted for this article.](/cms/asset/ad443820-460e-4dd7-9e91-abde33e55785/ktmp_a_1691896_f0010_oc.jpg)
Figure 11. Mean changes in brain (mPOA), skin, and body core temperature and temperature differentials during general anesthesia induced by sodium pentobarbital without and with body warming. Vertical hatched line = the moment of ip drug injection. Filled symbols show values significantly different from baseline (ANOVA followed by Fisher test p < 0.05). Data were replotted from reference [Citation119].
![Figure 11. Mean changes in brain (mPOA), skin, and body core temperature and temperature differentials during general anesthesia induced by sodium pentobarbital without and with body warming. Vertical hatched line = the moment of ip drug injection. Filled symbols show values significantly different from baseline (ANOVA followed by Fisher test p < 0.05). Data were replotted from reference [Citation119].](/cms/asset/81866ce4-ebe9-4af4-b676-f6c4a6b29585/ktmp_a_1691896_f0011_oc.jpg)
Figure 12. Original record of changes in temperature in the brain (mPOA, Hippo), skin, and arterial blood during arousing stimulation (TP, tail-pinch and SI, social interaction) and general anesthesia induced by sodium pentobarbital. Data were obtained in 2007 and previously not published.
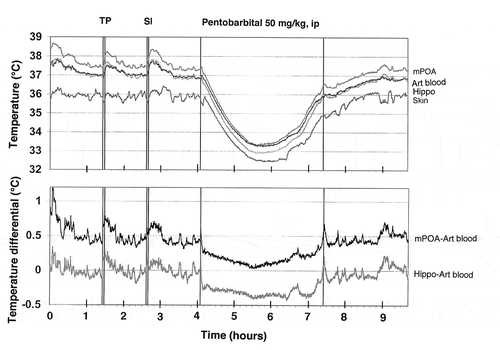
Figure 13. Temperature dependence of albumin immunoreactivity (Albumin), GFAP immunoreactivity (Glial Activation), and cellular brain abnormalities (Abnormal Cells) in pentobarbital-anesthetized rats passively warmed to different temperatures. Data on top graphs show values for four brain structures and data on bottom graphs show values for individual cortical areas. Values on abscissa show values of NAc temperature at which brain samples were taken. Data were replotted from reference [Citation158].
![Figure 13. Temperature dependence of albumin immunoreactivity (Albumin), GFAP immunoreactivity (Glial Activation), and cellular brain abnormalities (Abnormal Cells) in pentobarbital-anesthetized rats passively warmed to different temperatures. Data on top graphs show values for four brain structures and data on bottom graphs show values for individual cortical areas. Values on abscissa show values of NAc temperature at which brain samples were taken. Data were replotted from reference [Citation158].](/cms/asset/417c331a-77b5-4735-b548-4409b75019e2/ktmp_a_1691896_f0013_oc.jpg)
Figure 14. Temperature dependence of tissue water in the cortex and medial thalamus in pentobarbital-anesthetized rats passively warmed to different temperatures. Horizontal hatched lines show “normal” values evaluated in brains of awake, drug-free rats at normothermia. In each structure water tissue content tightly and linearly correlates with brain temperature (p < 0.001). Data were re-plotted from reference [Citation158].
![Figure 14. Temperature dependence of tissue water in the cortex and medial thalamus in pentobarbital-anesthetized rats passively warmed to different temperatures. Horizontal hatched lines show “normal” values evaluated in brains of awake, drug-free rats at normothermia. In each structure water tissue content tightly and linearly correlates with brain temperature (p < 0.001). Data were re-plotted from reference [Citation158].](/cms/asset/da05fe6a-15cd-4cd2-96d1-7597234c797a/ktmp_a_1691896_f0014_oc.jpg)
Figure 15. Correlative relationships between individual brain parameters in pentobarbital-anesthetized rats passively warmed to different temperatures. A = relationships between the counts of albumin- and GFAP-positive cells evaluated in the thalamus and cortex; B = relationships between the counts of albumin-positive cells and tissue water content; C = relationships between the counts of albumin-positive cells and structurally abnormal cells; D = relationships between tissue water content and the counts of albumin-positive cells. Each correlation coefficient value is highly significant (p < 0.001). See the text for other explanations. Original data were published in reference [Citation158] and re-plotted for this article.
![Figure 15. Correlative relationships between individual brain parameters in pentobarbital-anesthetized rats passively warmed to different temperatures. A = relationships between the counts of albumin- and GFAP-positive cells evaluated in the thalamus and cortex; B = relationships between the counts of albumin-positive cells and tissue water content; C = relationships between the counts of albumin-positive cells and structurally abnormal cells; D = relationships between tissue water content and the counts of albumin-positive cells. Each correlation coefficient value is highly significant (p < 0.001). See the text for other explanations. Original data were published in reference [Citation158] and re-plotted for this article.](/cms/asset/7e31ad68-f727-4341-802d-ec665ac35842/ktmp_a_1691896_f0015_oc.jpg)
Figure 16. Temperature dependence of structural brain changes. Left pictures show Nissl-stained sections from similar areas of the deep parietal cortex obtained from rats maintained at different levels of NAc temperature (S15 = 36.05°C; S29 = 32.30°C; S12 = 41.80°C). Brain cells during hyperthermia have larger somata and wider axons (arrows) compared to those in normothermia. In contrast, during hypothermia cells are slightly smaller in size, staining is more condensed, and axons are smaller in diameter compared to normothermic conditions (arrows). Bar for each graph is 100 μm. Right pictures show profound changes in structural integrity of the choroid plexus during extreme hyperthermia. All slices were made over lateral ventricles, stained with Hematoxylin-Eosin, and images are shown with equal magnification (bar = 200 μm). In contrast to the healthy structure in normothermic conditions, robust disintegration of epithelial cells and profound vacuolization were typical of hyperthermic conditions. Modified from reference [Citation158].
![Figure 16. Temperature dependence of structural brain changes. Left pictures show Nissl-stained sections from similar areas of the deep parietal cortex obtained from rats maintained at different levels of NAc temperature (S15 = 36.05°C; S29 = 32.30°C; S12 = 41.80°C). Brain cells during hyperthermia have larger somata and wider axons (arrows) compared to those in normothermia. In contrast, during hypothermia cells are slightly smaller in size, staining is more condensed, and axons are smaller in diameter compared to normothermic conditions (arrows). Bar for each graph is 100 μm. Right pictures show profound changes in structural integrity of the choroid plexus during extreme hyperthermia. All slices were made over lateral ventricles, stained with Hematoxylin-Eosin, and images are shown with equal magnification (bar = 200 μm). In contrast to the healthy structure in normothermic conditions, robust disintegration of epithelial cells and profound vacuolization were typical of hyperthermic conditions. Modified from reference [Citation158].](/cms/asset/d1f13db6-a895-4723-8757-556d19d940be/ktmp_a_1691896_f0016_oc.jpg)
Figure 17. In vitro current responses of GLU- (A) and glucose- (B) selective (Active) and substrate-null (Null) biosensors to different concentrations of substrates before (Pre) and after (Post) an in vivo recording. (C) Current response of an example GLU biosensor at 20°C versus 37°C. Dashed lines represent the baseline current in vitro at each temperature, each arrow indicates the time when 10 µM GLU was added. Note that the baseline at 37°C is approximately equivalent to the current response to 10 µM GLU at 20°C. Right bar graph indicates the percent increase in current between 20°C and 37°C for the GLU and glucose sensors tested. Original data were published in [Citation174,Citation177]; the picture was reproduced in [Citation187] (ACS Chemical Neuroscience; open access publication).
![Figure 17. In vitro current responses of GLU- (A) and glucose- (B) selective (Active) and substrate-null (Null) biosensors to different concentrations of substrates before (Pre) and after (Post) an in vivo recording. (C) Current response of an example GLU biosensor at 20°C versus 37°C. Dashed lines represent the baseline current in vitro at each temperature, each arrow indicates the time when 10 µM GLU was added. Note that the baseline at 37°C is approximately equivalent to the current response to 10 µM GLU at 20°C. Right bar graph indicates the percent increase in current between 20°C and 37°C for the GLU and glucose sensors tested. Original data were published in [Citation174,Citation177]; the picture was reproduced in [Citation187] (ACS Chemical Neuroscience; open access publication).](/cms/asset/3e02baa1-2395-4630-82d1-f261b1ae368e/ktmp_a_1691896_f0017_oc.jpg)
Figure 18. Slow changes (mean±sem) in electrochemical currents detected in the NAc by substrate-specific and substrate-null sensors during long-term recordings in freely moving rats. Both GLU (A) and glucose (B) currents slowly decreased during ~8-hour in vivo recording. A similar decrease, but at lower absolute levels, occurred with both types of null sensors recorded during the same time in vitro at 22–23°C. Since active sensors differ from null sensors only by the presence of a specific enzyme (glutamate oxidase or glucose oxidase), the difference in currents detected by these sensors reflects the GLU or glucose contribution (red vertical lines in A and B). Open and close circles in A show current values in the NAc shell and core, while a solid line shows their average. C compares the proportion of the specific contribution of GLU and glucose (in red) with respect to nonspecific contributions (gray and blue) to the overall recorded current. While these nonspecific contributions are similar for both glutamate and glucose sensors, the specific component is much larger for glucose than for glutamate. Original data were published in [Citation174,Citation177]; the picture was reproduced in [Citation187] (ACS Chemical Neuroscience; open access publication).
![Figure 18. Slow changes (mean±sem) in electrochemical currents detected in the NAc by substrate-specific and substrate-null sensors during long-term recordings in freely moving rats. Both GLU (A) and glucose (B) currents slowly decreased during ~8-hour in vivo recording. A similar decrease, but at lower absolute levels, occurred with both types of null sensors recorded during the same time in vitro at 22–23°C. Since active sensors differ from null sensors only by the presence of a specific enzyme (glutamate oxidase or glucose oxidase), the difference in currents detected by these sensors reflects the GLU or glucose contribution (red vertical lines in A and B). Open and close circles in A show current values in the NAc shell and core, while a solid line shows their average. C compares the proportion of the specific contribution of GLU and glucose (in red) with respect to nonspecific contributions (gray and blue) to the overall recorded current. While these nonspecific contributions are similar for both glutamate and glucose sensors, the specific component is much larger for glucose than for glutamate. Original data were published in [Citation174,Citation177]; the picture was reproduced in [Citation187] (ACS Chemical Neuroscience; open access publication).](/cms/asset/a689c67a-30b9-4179-b231-02c52c408ab6/ktmp_a_1691896_f0018_oc.jpg)
Figure 19. Slow changes in electrochemical currents detected by GLU and GLU-null sensors in the NAc shell (left panel) and NAc core (right panel) during a 3-min tail-pinch in freely moving rats. Top row (A and C) shows mean (±SEM) changes in total currents detected by both types of sensors. Middle row (B and D) shows current differences (active – null) calibrated in nM GLU concentrations. The two vertical hatched lines in each graph show onset and offset of tail-pinch and horizontal hatched lines show baselines. Two diagonal lines in A show the trend in current baselines calculated for GLU and GLU-null sensors. Asterisks in A show the period of significant differences in currents (current x time interaction assessed with two-way ANOVA) detected by active and null sensors. Significant differences in currents (active – null) are shown in B as filled symbols. E shows mean(±SEM) changes in NAc temperature and electrochemical current detected by GLU-null sensors during 3-min tail-pinch. Original data were published in [Citation174,Citation177]; the picture was reproduced in [Citation187] (ACS Chemical Neuroscience; open access publication).
![Figure 19. Slow changes in electrochemical currents detected by GLU and GLU-null sensors in the NAc shell (left panel) and NAc core (right panel) during a 3-min tail-pinch in freely moving rats. Top row (A and C) shows mean (±SEM) changes in total currents detected by both types of sensors. Middle row (B and D) shows current differences (active – null) calibrated in nM GLU concentrations. The two vertical hatched lines in each graph show onset and offset of tail-pinch and horizontal hatched lines show baselines. Two diagonal lines in A show the trend in current baselines calculated for GLU and GLU-null sensors. Asterisks in A show the period of significant differences in currents (current x time interaction assessed with two-way ANOVA) detected by active and null sensors. Significant differences in currents (active – null) are shown in B as filled symbols. E shows mean(±SEM) changes in NAc temperature and electrochemical current detected by GLU-null sensors during 3-min tail-pinch. Original data were published in [Citation174,Citation177]; the picture was reproduced in [Citation187] (ACS Chemical Neuroscience; open access publication).](/cms/asset/acab47d8-7afb-48ef-8b7f-5884091109b5/ktmp_a_1691896_f0019_oc.jpg)
Figure 20. Mean (±SEM) changes in electrochemical currents detected by glucose and glucose-null sensors (A, C, E) and resulting changes in [glucose] (B, D, F induced by different sensory stimuli in freely moving rats. Data for audio stimulus and novel object are shown with 2-s time resolution, and tail-touch shown with 4-s time resolution. Original data were published in [Citation175]; the picture was reproduced in [Citation187] (ACS Chemical Neuroscience; open access publication).
![Figure 20. Mean (±SEM) changes in electrochemical currents detected by glucose and glucose-null sensors (A, C, E) and resulting changes in [glucose] (B, D, F induced by different sensory stimuli in freely moving rats. Data for audio stimulus and novel object are shown with 2-s time resolution, and tail-touch shown with 4-s time resolution. Original data were published in [Citation175]; the picture was reproduced in [Citation187] (ACS Chemical Neuroscience; open access publication).](/cms/asset/799de941-6d13-4ab1-8780-9230e854d465/ktmp_a_1691896_f0020_oc.jpg)
Figure 21. Changes in NAc, muscle, and skin temperature induced by iv cocaine (1 mg/kg) in freely moving rats. A = temperature changes vs. pre-injection baseline; B = temperature differentials; C = locomotor activity; D = relationships between the magnitude of cocaine-induced NAc temperature elevation and basal temperature; n = number of analyzed responses; r = coefficient of correlation. Regression line crosses the line of no effect at ~39.5°C. Data were replotted from reference [Citation222].
![Figure 21. Changes in NAc, muscle, and skin temperature induced by iv cocaine (1 mg/kg) in freely moving rats. A = temperature changes vs. pre-injection baseline; B = temperature differentials; C = locomotor activity; D = relationships between the magnitude of cocaine-induced NAc temperature elevation and basal temperature; n = number of analyzed responses; r = coefficient of correlation. Regression line crosses the line of no effect at ~39.5°C. Data were replotted from reference [Citation222].](/cms/asset/b93ef537-53ce-43e4-b860-726f291c4bbd/ktmp_a_1691896_f0021_oc.jpg)
Figure 22. Changes in brain (NAc, nucleus accumbens; VTA, ventral tegmental area of midbrain, Hippo, hippocampus) and muscle temperatures during cocaine self-administration in trained rats. A and B = changes in absolute and relative temperatures averaged for each consecutive cocaine self-injection. L + S, the moment of light+sound presentation, when the lever became accessible and the rat could press a lever. C = temperature changes associated with the first-in-session cocaine self-injection. D = temperature changes associated with regular cocaine self-injections. E = differences in NAc temperature changes after a typical single-dose (1.0 mg/kg) and double-dose (2.0 mg/kg) cocaine self-injections. Data were replotted from [Citation223].
![Figure 22. Changes in brain (NAc, nucleus accumbens; VTA, ventral tegmental area of midbrain, Hippo, hippocampus) and muscle temperatures during cocaine self-administration in trained rats. A and B = changes in absolute and relative temperatures averaged for each consecutive cocaine self-injection. L + S, the moment of light+sound presentation, when the lever became accessible and the rat could press a lever. C = temperature changes associated with the first-in-session cocaine self-injection. D = temperature changes associated with regular cocaine self-injections. E = differences in NAc temperature changes after a typical single-dose (1.0 mg/kg) and double-dose (2.0 mg/kg) cocaine self-injections. Data were replotted from [Citation223].](/cms/asset/14d5a4a3-5552-4503-a6a9-adc0959d557e/ktmp_a_1691896_f0022_b.gif)
Figure 23. Changes in NAc temperature associated with the initial cocaine self-injection of a session (A), subsequent, regular self-injections (B) and the last self-injection of a session, when the operant level was blocked and drug was unavailable (filled symbols). Each graph also shows the changes associated with the same events when cocaine was injected passively (yoked-control) (open circles). Data were replotted from [Citation223,Citation224].
![Figure 23. Changes in NAc temperature associated with the initial cocaine self-injection of a session (A), subsequent, regular self-injections (B) and the last self-injection of a session, when the operant level was blocked and drug was unavailable (filled symbols). Each graph also shows the changes associated with the same events when cocaine was injected passively (yoked-control) (open circles). Data were replotted from [Citation223,Citation224].](/cms/asset/cc7ee6dd-beb4-465d-bf8e-dd532b731461/ktmp_a_1691896_f0023_b.gif)
Figure 24. Behavioral contribution to NAc temperature changes during cocaine self-administration in trained rats. Top graph (A, black symbols) shows mean values of NAc temperature immediately before each critical behavioral event (CS, light+sound conditioned cue, arrows with numbers are consecutive cocaine self-injections. White symbols shown NAc temperature values associated with the same events in yoked-control animals. B shows differences in NAc temperature between active and passive administration of cocaine. Filled triangles show values with significant between-group differences. Picture is based on re-analysis of original data presented in references [Citation223,Citation224].
![Figure 24. Behavioral contribution to NAc temperature changes during cocaine self-administration in trained rats. Top graph (A, black symbols) shows mean values of NAc temperature immediately before each critical behavioral event (CS, light+sound conditioned cue, arrows with numbers are consecutive cocaine self-injections. White symbols shown NAc temperature values associated with the same events in yoked-control animals. B shows differences in NAc temperature between active and passive administration of cocaine. Filled triangles show values with significant between-group differences. Picture is based on re-analysis of original data presented in references [Citation223,Citation224].](/cms/asset/aeabf1ec-4a08-4059-bfe4-65fc93c4b927/ktmp_a_1691896_f0024_oc.jpg)
Figure 25. Changes in NAc, muscle, and skin temperature induced by iv heroin at different doses in freely moving rats. At the smallest dose that is optimal for drug self-administration, heroin induces monophasic increases in brain and muscle temperature. However, with increase in doses, the response became biphasic, with the initial, dose-dependent temperature decreases and subsequent temperature increases. Data were replotted from [Citation228].
![Figure 25. Changes in NAc, muscle, and skin temperature induced by iv heroin at different doses in freely moving rats. At the smallest dose that is optimal for drug self-administration, heroin induces monophasic increases in brain and muscle temperature. However, with increase in doses, the response became biphasic, with the initial, dose-dependent temperature decreases and subsequent temperature increases. Data were replotted from [Citation228].](/cms/asset/594371a7-69e0-49f8-8325-02c5b22d8479/ktmp_a_1691896_f0025_oc.jpg)
Figure 26. State-dependency and environmental modulation of temperature responses induced by iv heroin (0.1 mg/kg) in freely moving rats. When heroin as injected during social interaction (yellow area from 0 to 60 min), temperature elevation and decrease in skin-muscle differentials became larger compared to the effects induced by this dug in quiet resting conditions. When heroin was injected at 29·C ambient temperatures, drug-induced temperature elevation became more prolonged. Data were replotted from [Citation228].
![Figure 26. State-dependency and environmental modulation of temperature responses induced by iv heroin (0.1 mg/kg) in freely moving rats. When heroin as injected during social interaction (yellow area from 0 to 60 min), temperature elevation and decrease in skin-muscle differentials became larger compared to the effects induced by this dug in quiet resting conditions. When heroin was injected at 29·C ambient temperatures, drug-induced temperature elevation became more prolonged. Data were replotted from [Citation228].](/cms/asset/811c6979-8a32-42de-be7b-829da7c8cbf9/ktmp_a_1691896_f0026_oc.jpg)
Figure 27. Changes (mean±SEM) in brain (NAc, nucleus accumbens) and muscle temperatures during heroin self-administration in trained rats. A and B = changes in absolute and relative temperatures averaged for each consecutive drug self-injection of a session. L + S = the moment of light+sound presentation, when the lever became accessible and the rat could press a lever. Asterisks show values significantly different from the previous value. C = temperature changes associated with the first-in-session heroin SA (arrow). D = temperature changes associated with regular heroin self-injections (filled symbols indicate values significantly different from the last pre-lever-press value (hatched line). E = differences in temperature changes after a typical single-dose (0.1 mg/kg) and double-dose (0.2 mg/kg) heroin self-administrations. Data were replotted from [Citation229].
![Figure 27. Changes (mean±SEM) in brain (NAc, nucleus accumbens) and muscle temperatures during heroin self-administration in trained rats. A and B = changes in absolute and relative temperatures averaged for each consecutive drug self-injection of a session. L + S = the moment of light+sound presentation, when the lever became accessible and the rat could press a lever. Asterisks show values significantly different from the previous value. C = temperature changes associated with the first-in-session heroin SA (arrow). D = temperature changes associated with regular heroin self-injections (filled symbols indicate values significantly different from the last pre-lever-press value (hatched line). E = differences in temperature changes after a typical single-dose (0.1 mg/kg) and double-dose (0.2 mg/kg) heroin self-administrations. Data were replotted from [Citation229].](/cms/asset/171c2959-0727-47d7-9494-39d05f08cefb/ktmp_a_1691896_f0027_b.gif)
Figure 28. A and B. Mean (±SEM) changes in NAc and muscle temperature and NAc-muscle temperature differentials following exposure to light+sound stimulus that was previously paired with heroin self-injections (conditioned sensory cue A) and preceding the first heroin self-injection of a session (B) in trained rats. Filled symbols show values significantly different from baseline (the last minute before the event). C. Changes in NAc-muscle temperature differentials during heroin self-administration session in trained rats. L + S = presentation of a conditioned sensory cue, symbols with numbers = consecutive heroin self-injections. Filled symbols show values significantly different from baseline (0 min). Data were replotted from [Citation229].
![Figure 28. A and B. Mean (±SEM) changes in NAc and muscle temperature and NAc-muscle temperature differentials following exposure to light+sound stimulus that was previously paired with heroin self-injections (conditioned sensory cue A) and preceding the first heroin self-injection of a session (B) in trained rats. Filled symbols show values significantly different from baseline (the last minute before the event). C. Changes in NAc-muscle temperature differentials during heroin self-administration session in trained rats. L + S = presentation of a conditioned sensory cue, symbols with numbers = consecutive heroin self-injections. Filled symbols show values significantly different from baseline (0 min). Data were replotted from [Citation229].](/cms/asset/4519e9fa-afc9-4e0b-b475-9acc55295712/ktmp_a_1691896_f0028_b.gif)
Figure 29. Potentiation of brain and body hyperthermia induced by MDMA (9 mg/kg, sc) during social interaction. When MDMA was injected during quiet rest, it induced a relatively modest increase in NAc and muscle temperature. This response was strongly potentiated when MDMA was injected 10-min after the start of social interaction, which by itself induced strong but transient increases in NAc and muscle temperatures. MDMA in each condition induced increases in NAc-muscle and decreases in skin-muscle differentials. During social interaction, both effects increase, with especially strong and prolonged decrease in skin-muscle differentials, suggesting sustained skin vasoconstriction. Original data were published in [Citation237] (Journal of Neuroscience, open access).
![Figure 29. Potentiation of brain and body hyperthermia induced by MDMA (9 mg/kg, sc) during social interaction. When MDMA was injected during quiet rest, it induced a relatively modest increase in NAc and muscle temperature. This response was strongly potentiated when MDMA was injected 10-min after the start of social interaction, which by itself induced strong but transient increases in NAc and muscle temperatures. MDMA in each condition induced increases in NAc-muscle and decreases in skin-muscle differentials. During social interaction, both effects increase, with especially strong and prolonged decrease in skin-muscle differentials, suggesting sustained skin vasoconstriction. Original data were published in [Citation237] (Journal of Neuroscience, open access).](/cms/asset/52bc2a98-e874-4bfd-a21d-51c5fae45ee0/ktmp_a_1691896_f0029_oc.jpg)
Figure 30. MDMA injected at a dose ~1/5 of the LD50 (9 mg/kg) at normothermic conditions (29°C) induces lethality in all tested rats. A = NAc temperature changes in individual animals; B = changes in NAc, muscle, and skin temperatures for the first 90 min from MDMA injection, when all rats were still alive; C = changes in NAc-muscle and skin-muscle differentials; D = changes in locomotor activity, E = changes in NAc-muscle differentials preceding and following termination of breathing in individual rats; F = mean changes in NAc-muscle and skin-muscle differentials preceding and following termination of breathing. Bar graphs show differences in temperature and locomotor effects for 90 min after MDMA injection at 23°C and 29°C. Original data were published in [Citation237] (Journal of Neuroscience, open access).
![Figure 30. MDMA injected at a dose ~1/5 of the LD50 (9 mg/kg) at normothermic conditions (29°C) induces lethality in all tested rats. A = NAc temperature changes in individual animals; B = changes in NAc, muscle, and skin temperatures for the first 90 min from MDMA injection, when all rats were still alive; C = changes in NAc-muscle and skin-muscle differentials; D = changes in locomotor activity, E = changes in NAc-muscle differentials preceding and following termination of breathing in individual rats; F = mean changes in NAc-muscle and skin-muscle differentials preceding and following termination of breathing. Bar graphs show differences in temperature and locomotor effects for 90 min after MDMA injection at 23°C and 29°C. Original data were published in [Citation237] (Journal of Neuroscience, open access).](/cms/asset/8c7286bd-8955-436f-96f3-751e0ce768fd/ktmp_a_1691896_f0030_oc.jpg)

