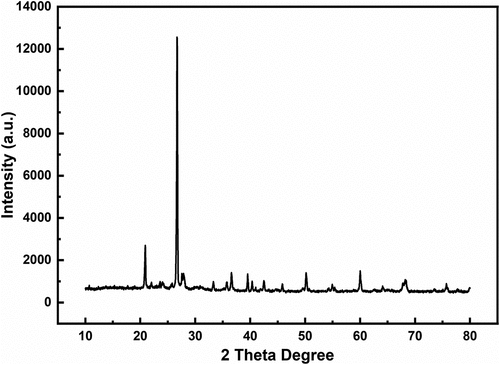
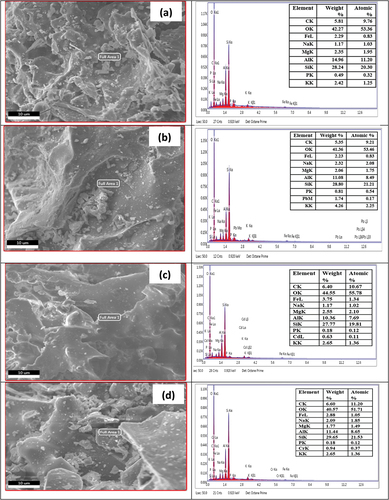
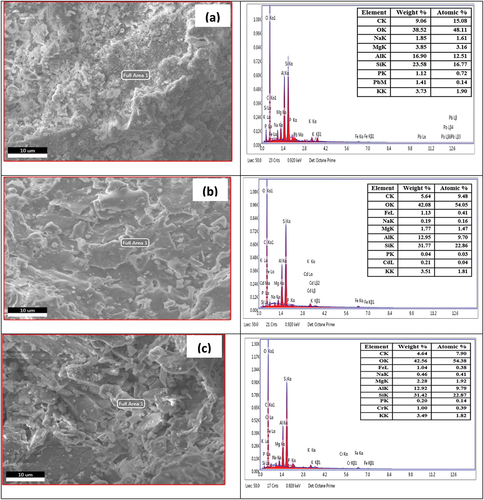
Figures & data
Figure 2. a) removal of heavy metals (pb, Cd & Cr) using untreated and b) removal of heavy metals (pb, Cd & Cr) using treated brick nano particles as an adsorbent with respect to time.
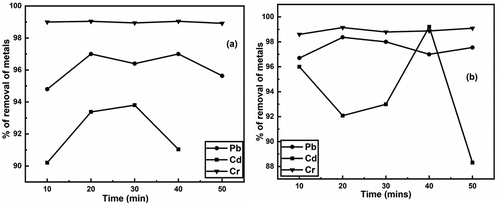
Figure 3. a) removal of heavy metals (pb, Cd & Cr) using untreated and b) removal of heavy metals (pb, Cd & Cr) using treated brick nano particles as an adsorbent with respect to temperature.
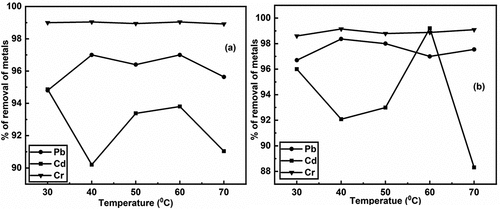
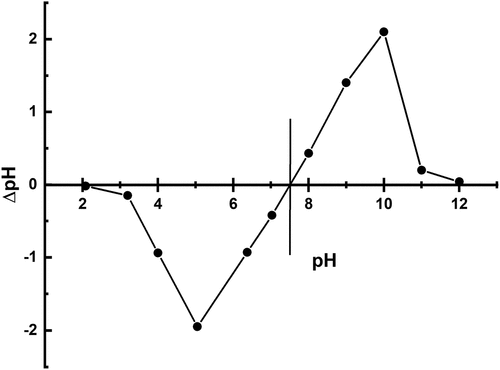
Figure 4. a) removal of heavy metals (pb, Cd & Cr) using untreated and b) removal of heavy metals (pb, Cd & Cr) using treated brick nano particles as an adsorbent with respect to pH.
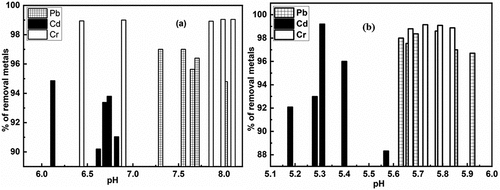
Figure 6. Fitting of Longmuir adsorption isotherm model for removal of heavy metals using untreated and treated brick sand nanoparticles for Pb [(a) &; (b)], Cd [(c) &; (d)] and Cr [(e) &; (f)].
![Figure 6. Fitting of Longmuir adsorption isotherm model for removal of heavy metals using untreated and treated brick sand nanoparticles for Pb [(a) &; (b)], Cd [(c) &; (d)] and Cr [(e) &; (f)].](/cms/asset/28463e90-a705-4adb-b4cf-50004b6bab3f/twas_a_2307197_f0006_b.gif)
Table 1. Summary of the three-adsorption model used in the study for removal of heavy toxic metals (pb, Cd & Cr) from aqueous solution using brick sand nanoparticles as an adsorbent.
Figure 7. Fitting of Freundlich adsorption isotherm model for removal of heavy metals using untreated and treated brick sand nanoparticles for Pb [(a) &; (b)], Cd [(c) &; (d)] and Cr [(e) &; (f)].
![Figure 7. Fitting of Freundlich adsorption isotherm model for removal of heavy metals using untreated and treated brick sand nanoparticles for Pb [(a) &; (b)], Cd [(c) &; (d)] and Cr [(e) &; (f)].](/cms/asset/83beebff-31be-421f-bad6-b355547a70f7/twas_a_2307197_f0007_b.gif)
Figure 8. Fitting of Temkin adsorption isotherm model for removal of heavy metals using untreated and treated brick sand nanoparticles for Pb [(a) &; (b)], Cd [(c) &; (d)] and Cr [(e) &; (f)].
![Figure 8. Fitting of Temkin adsorption isotherm model for removal of heavy metals using untreated and treated brick sand nanoparticles for Pb [(a) &; (b)], Cd [(c) &; (d)] and Cr [(e) &; (f)].](/cms/asset/d0ed0dde-1121-4d66-b480-471ca60c987d/twas_a_2307197_f0008_b.gif)
Figure 9. Fitting of Hill De Boer adsorption isotherm model for removal of heavy metals using untreated and treated brick sand nanoparticles for Pb, Cd, and Cr.
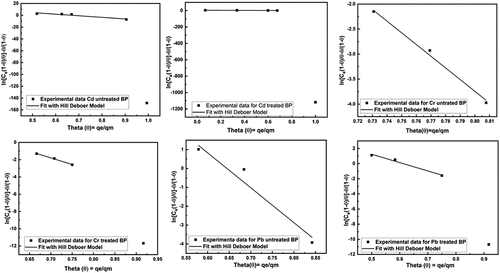
Table 2. Summary of the hill de boer, elovich and redlich-petersons adsorption model used in the study for removal of heavy toxic metals (pb, Cd & Cr) from aqueous solution using brick sand nanoparticles as an adsorbent.
Figure 10. Fitting of Elovich adsorption isotherm model for removal of heavy metals using untreated and treated brick sand nanoparticles for Pb, Cd, and Cr.
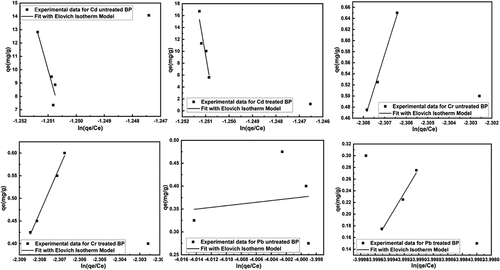
Figure 11. Fitting of redlich-petersons adsorption isotherm model for removal of heavy metals using untreated and treated brick sand nanoparticles for Pb, Cd, and Cr.
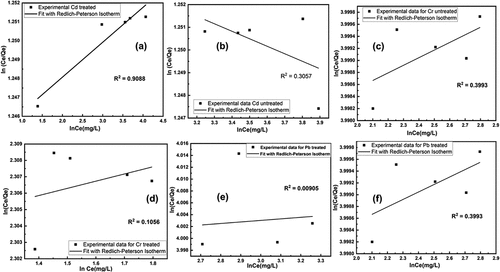
Figure 12. Fitting of Intra-particle diffusion model for removal of heavy metals using untreated and treated brick sand nanoparticles for Pb, Cd, and Cr.
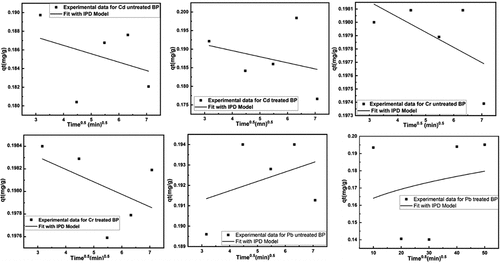
Table 3. Summary of the intra-particle diffusion, elovich and boyd kinetic model used in the study for removal of heavy toxic metals (pb, Cd & Cr) from aqueous solution using brick sand nanoparticles as an adsorbent.
Figure 13. Fitting of Elovich kinetic model for removal of heavy metals using untreated and treated brick sand nanoparticles for Pb, Cd, and Cr.
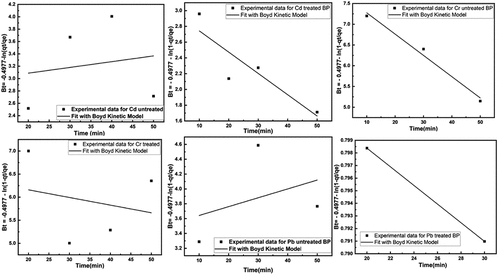
Figure 14. Fitting of Boyd model for removal of heavy metals using untreated and treated brick sand nanoparticles for Pb, Cd, and Cr.
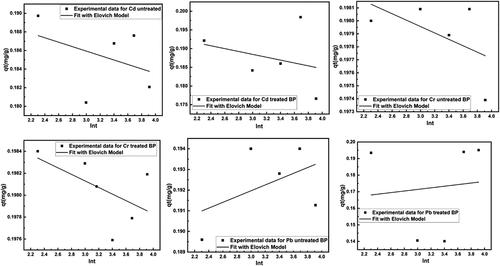
Figure 15. Kinetic for removal of heavy metals using untreated and treated brick sand nanoparticles for Pb [(a) &; (b)], Cd [(c) &; (d)] and Cr [(e) &; (f)].
![Figure 15. Kinetic for removal of heavy metals using untreated and treated brick sand nanoparticles for Pb [(a) &; (b)], Cd [(c) &; (d)] and Cr [(e) &; (f)].](/cms/asset/abd50729-69e8-4d17-a92d-769d2bf6f235/twas_a_2307197_f0015_b.gif)
Table 4. Pseudo 2nd order reaction kinetics parameter for untreated and treated brick sand nano-particles for removal of heavy toxic metals Pb, Cd, and Cr.
Table 5. Thermodynamics parameter for untreated and treated brick sand nano-particles (pb, Cd, and Cr) using R = 8.316 Jmol−1K−1..