Figures & data
Figure 1. Development of the pathologic component scoring system and evaluation for prognostic value and reproducibility.
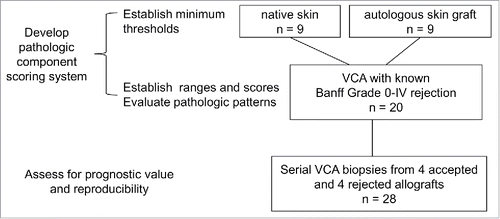
Table 1. Definitions of pathological components and scores.
Table 2. Pathologic component scores of first biopsies between 2 groups.
Figure 2. Number of epidermal mononuclear cells per 4 medium power (20x) fields in native and autologous skin flaps
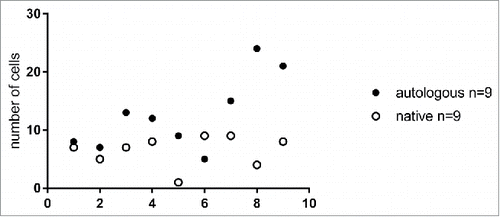
Figure 3. Pathologic component scores across 20 VCA biopsies with different Banff grades of rejection.
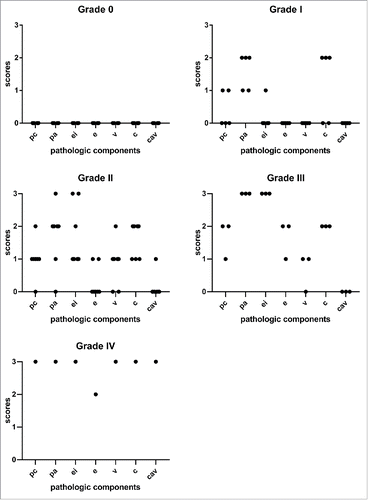
Figure 4. Histologic features of porcine skin and skin-containing VCA and pathologic component scores. A) Normal pig skin, punch biopsy. B) Minimal perivascular infiltrate (pc0) with capillaritis (c1) (arrows) in the superficial dermis. C) Moderately dense perivascular inflammation (pc2) with more evident capillaritis (c2) (arrows) at 40x magnification. D) The perivascular infiltrate surrounds dilated superficial dermal capillaries (pc1) occupying more than half of the field at 40x magnification (pa2) (outlined in blue). Dilated capillaries show occasional intraluminal mononuclear cells (c1) (arrows).
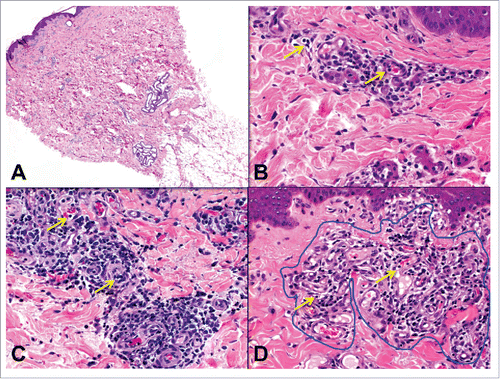
Figure 5. Epidermal inflammation (ei) and injury (e) in skin-containing VCA. A) Epidermis at 40x magnification with no epidermal infiltrate (ei0). Dilated superficial dermal capillaries with minimal perivascular inflammation are also present. B) One focus of prominent epidermal involvement without apoptosis (ei3, e0). C and D) Focal keratinocyte apoptosis (e1) (arrows). E) Frank epidermal necrosis. Moderate perivascular inflammation in the superficial dermis is also present.
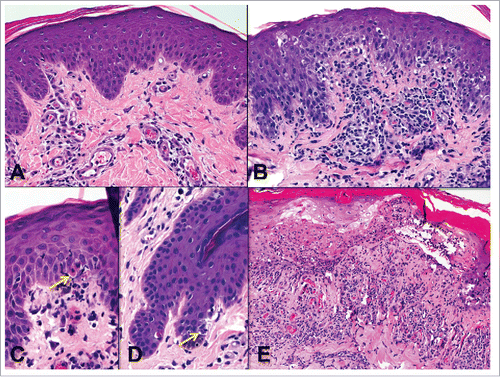
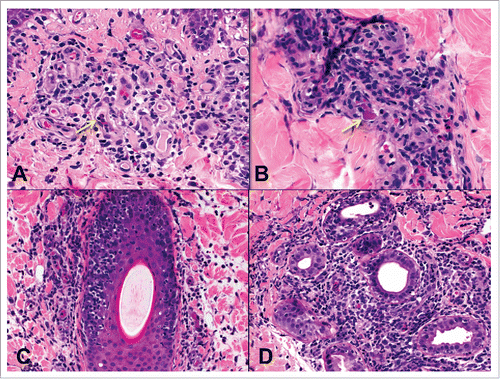
Figure 6. Endothelialitis (v) and chronic allograft vasculopathy (cav). A) Small artery with endothelialitis (v1) (arrow). B) Artery with endothelialitis (arrow) and transmural inflammation in an arteriole (arrowheads) (v3). C) Transmural arteritis (v3). Fibrin (arrows) can be seen in v lesions. D) Early chronic allograft vasculopathy (cav1) in a small artery. E) Severe chronic allograft vasculopathy with luminal reduction (cav3).
Figure 7. Pathologic component scores across accepted and rejected allografts over time (in days). Grafts that rejected and survived for less than 100 d (black circles) have higher pathologic component scores than grafts that were accepted (white circles).
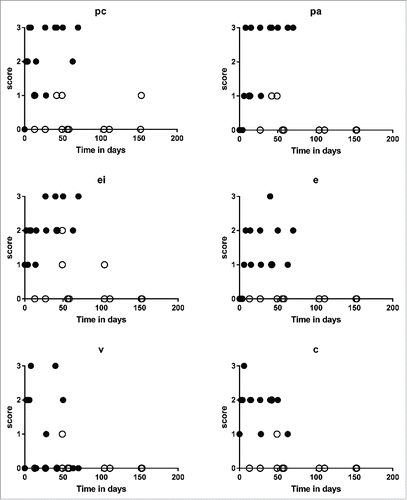
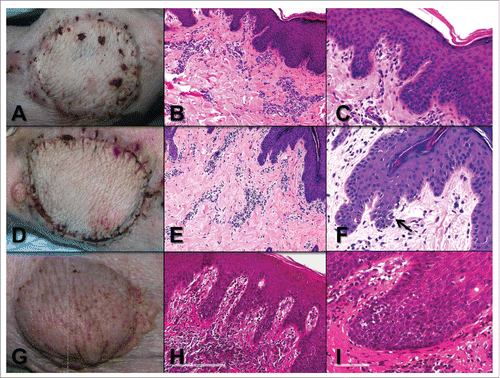
Figure 8. A) Animal A3 VCA post-surgical day 13 showing mild edema. B) Animal A3 POD 13 skin biopsy showing minimal perivascular inflammation (pc1, pa1) and C) no epidermal involvement (ei0, e0). D) Animal B3 POD 14 showing mild edema. E) Animal B3 POD 14 skin punch biopsy showing mild perivascular inflammation (pc1, pa1) but with F) epidermal apoptosis (arrow) and focal necrosis (not shown) (ei1, e2). G) Animal B1 POD 42 showing erythema. H) Animal B1 POD 42 skin biopsy showing severe perivascular inflammation (pc3, pa3) and I) lymphocytic infiltration and focal apoptosis (not shown) (ei2, e1).