Figures & data
Figure 1. Pathways involved in NAD+ biosynthesis and catabolism. Metabolites: NA, nicotinic acid or niacin; TRP, tryptophan; QA, quinolinic acid; NAMN, NA mononucleotide; NAAD, nicotinic acid adenine dinucleotide; NAD+, nicotinamide adenine dinucleotide (oxidized); NM, nicotinamide; NMN, NM mononucleotide; NR, nicotinamide riboside. Metabolic enzymes are: NAPRT1, nicotinic acid phosphoribosyltransferase; NMNAT1,2,3, nicotinamide nucleotide adenylyltransferases; NADS, NAD+ synthetase; PARPs, poly ADP-ribose polymerases; SIRTs, sirtuins; CD38, cluster of differentiation 38 or cyclic ADP-ribose hydrolase; NAMPT, nicotinamide phosphoribosyl transferase; NRK1,2, nicotinamide riboside kinases; Cx43, connexin43; CD73, cluster of differentiation 73 or ecto-5′-nucleotidase.
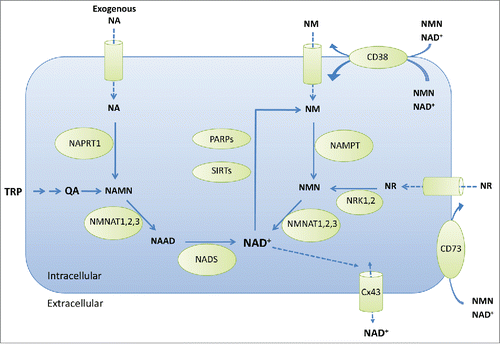
Figure 2. Mechanism of synergy between DNA damaging agents and NAMPT inhibitors. NAMPTi synergize with several DNA damaging agents, all of which have been shown to induce base excision repair (BER) or single-stranded DNA damage resulting in PARP activation. The increased consumption of NAD+ caused by PARP activation combined with inhibition of NAD+ regeneration leads to catastrophic NAD+ depletion and cell death. In cells that do not reach the threshold level/duration of NAD+ depletion, accumulation of unrepaired DNA may later lead to cell cycle arrest and tumor cell death. BER, base excision repair; NAD+, nicotinamide adenine dinucleotide (oxidized); NAMPT, nicotinamide phosphoribosyl transferase; NAMPTi, NAMPT inhibitor; PARP, poly ADP-ribose polymerase.
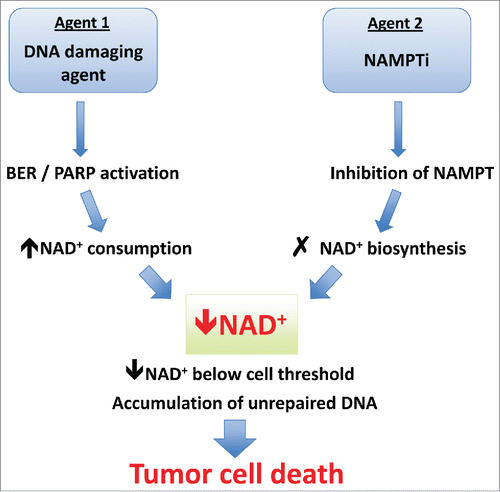
Table 1. Potential therapeutic strategies for NAMPT inhibitors involving drug combinations.
