Figures & data
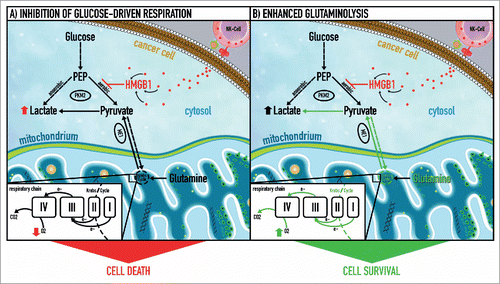
Figure 1. NK cell-derived HMGB1 controls glycolysis and aerobic respiration by allosteric inhibition of tetrameric pyruvate kinase M2. (1) High mobility group box 1 (HMGB1) protein induces a metabolic type of cell death in cancer cells via inhibition of tetrameric pyruvate kinase (PK) M2 and subsequent blockage of glucose-driven respiration. Thus, HMGB1 rapidly forces cancer cells to rely on glycolysis (employing dimeric PK M2) as the only remaining source of energy production. (2) A subgroup of cancer cells can evade HMGB1-induced metabolic cell death by switching to glutamine breakdown and thus preserving electron flux through the respiratory chain. The latter is coupled to oxidative phosphorylation and provides an alternative energy source that compensates for the energy deficit caused by inhibition of glucose-dependent aerobic respiration through HMGB1. This metabolic plasticity allows cancer cells to resist HMGB1-induced metabolic cell death. e−, electron flow; ME I-IV, malic enzyme complex I-IV; PEP, phosphoenolpyruvate; PK M2, pyruvate kinase isoform M2.