Figures & data
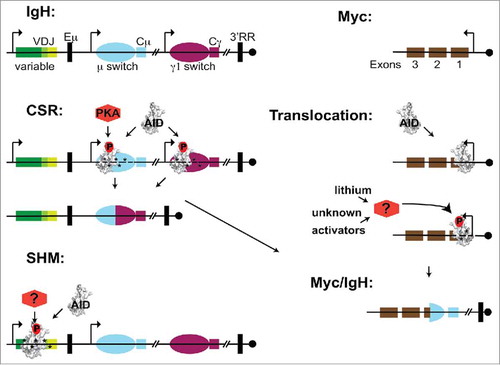
Figure 1. The danger of dysregulated activation-induced cytidine deaminase (AID) phosphorylation. Schematic of the Immunoglobulin heavy (Igh) locus: a recombined V(D)J and constant region exons (colored boxes), non-coding switch regions (ovals), regulatory elements (black boxes) including mu enhancer, downstream enhancers and 3′ regulatory region. The Myc exons (brown boxes) include non-coding exon 1 and centromere orientation (circle) is shown. (B) During class switch recombination (CSR), AID prominently associates with Igh switch regions. However, AID association is promiscuous and occurs at transcriptionally active genes across the genome such as Myc. Phosphorylation on AID serine 38 (AID-pS38) depicted as (red-P), induces mutator activity (*) which cause double strand breaks (DSBs) (gaps) that serve as recombination substrates between isotype switch regions. AID-S38 is a target site for the ubiquitously expressed cAMP-dependent protein kinase (PKA), which is recruited to the Igh switch regions providing a mechanism to induce pS38 and AID activity there. Normally, AID associated with off-target genes normally has minimal AID-pS38 levels. Lithium activation of an unknown kinase pathway induces AID-pS38 levels at off-target sites such as Myc. This results in mutation and DSBs that serve as translocation substrates between Myc and Igh regions. The example depicts a translocation into Exon 1 that brings the coding exons of Myc under the influence of Igh regulatory elements (black boxes) driving overexpression. For somatic hypermutation (SHM), neither the AID targeting mechanism nor the kinase that phosphorylates AID at the Igh variable regions have been defined.