Figures & data
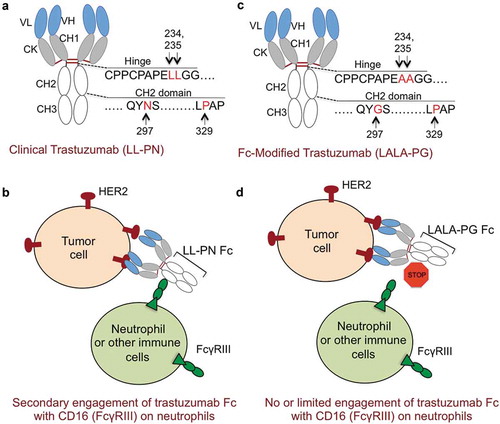
Figure 1. Improving the safety of trastuzumab and docetaxel combinations by limiting Fc-effector functions. (a) Schematic of clinical trastuzumab fragment crystallizable (Fc) region. Hinge domain of trastuzumab Fc has lysine (L) residues at positions 234, 235 and CH2 domain of Fc contains glycosylation site (asparagine: N) at 297 and proline (P) at 329 positions, respectively. This configuration is named as trastuzumab (LL-PN). (b) In addition to antibody directed cell cytotoxicity (ADCC) activating cluster of differentiation 16a (CD16a, also known as FcRγIIIa) receptor on natural killer (NK) cells, LL-PN Fc residues of trastuzumab have potential to engage high affinity CD16b isoform (FcγRIIIb) receptor on neutrophils (in Asian population) resulting in secondary and nonspecific engagement and toxicity to neutrophils. (c) Schematic of modified trastuzumab fragment crystallizable region (Fc) region. Hinge domain of Fc has 234 and 235 lysine’s (L) replaced with alanine (A) residues and CH2 domain of Fc contains asparagine (N) mutated to glycine (G). Proline (P) at position 329 remains unchanged. This configuration is named as trastuzumab (LALA-PG). (d) LALA-PG mutations in trastuzumab Fc will limit its ability to effectively engage FcRγIIIa and potentially FcRγIIIb receptors on neutrophils in Asian population.