Figures & data
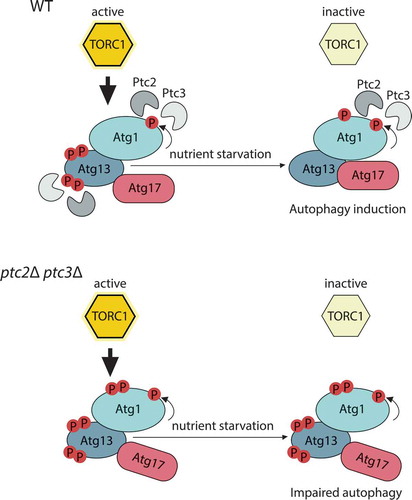
Figure 1. Ptc2 and Ptc3 promote autophagy through dephosphorylation of Atg1 kinase complex.
In wild-type (WT) cells, under nutrient-deplete conditions, target of rapamycin complex 1 (TORC1) is active, and Atg13 is kept in a hyperphosphorylated and inhibited state (upper panel). Atg1 kinase activity is stimulated by Atg11-bound cargo interactions, which lead to Atg1 phosphorylation. Ptc2 and Ptc3 counteract these modifications. Upon TORC1 inhibition by nutrient starvation, Ptc2 and Ptc3 contribute to the dephosphorylation of Atg13, and Atg1–Atg13–Atg17 complex formation, to allow proper autophagy induction. The deletion of these phosphatases (lower panel) leads to the accumulation of hyperphosphorylated Atg1 and Atg13 species and impaired Atg13–Atg17 interaction.