Figures & data
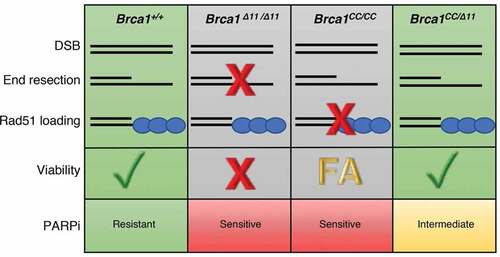
Figure 1. Link between homologous recombination functionality and development in Brca1 mutant mice. Wild-type Brca1 activity at DNA double strand breaks (DSB) supports the end resection and Rad51 loading steps of homologous recombination (HR), promoting viability and PARP inhibitor (PARPi) resistance. Brca1Δ11/Δ11 mouse embryonic fibroblasts (MEFs) produce the hypomorphic Brca1-Δ11 protein which does not promote end resection and results in dysfunctional HR, embryonic lethality, and sensitivity to PARPi. Brca1CC/CC MEFs express Brca1-CC and retain the ability to support end resection however fail to load Rad51 causing PARPi sensitivity, late embryonic lethality, and Fanconi anemia (FA)-like defects. In contrast, compound heterozygous Brca1CC/Δ11 mice exhibit resection and Rad51 loading due to the hypomorphic activities of Brca1-CC and Brca1-Δ11, respectively.