Figures & data
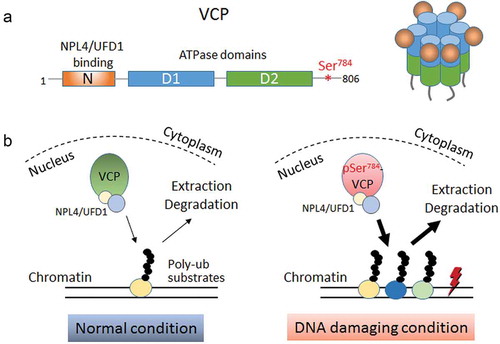
Figure 1. DNA damage-induced Ser784 phosphorylation selectively increases VCP (valosin-containing protein) activity for chromatin-associated protein degradation. (a) Schematics showing the domain structure of monomeric VCP (left) and 3D structure of a functional VCP hexamer (right). The N-terminal domain of VCP interacts with the majority of ubiquitin-binding cofactors such as NPL4 (Nuclear Protein Localization protein 4) and UFD1 (Ubiquitin recognition Factor in ER-associated Degradation 1). D1 and D2 are the central ATPase domains. Ser784 is located in the structurally disordered C-terminal tail of VCP. (b) Working model depicting the selective increase of nuclear VCP activity by DNA damage-induced Ser784 phosphorylation with regard to chromatin-associated protein degradation. In the absence of DNA damage, unphosphorylated VCP extracts its chromatin-associated poly-ubiquitinated substrates at a normal rate. Under DNA-damaging conditions, Ser784 phosphorylation turns VCP into a more efficient protein segregase presumably to extract more chromatin-associated substrates that are functionally important for DNA damage response.