Figures & data
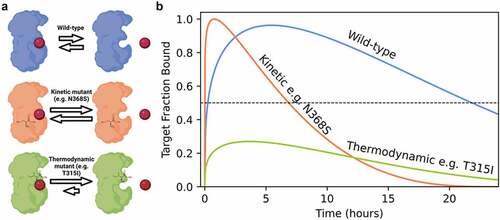
Figure 1. Altering drug binding rates may be a partial resistance mechanism to kinase inhibition. (a) Kinetic mutations in breakpoint cluster region (BCR)-ABL cause resistance through increased drug binding and dissociation rates, whereas thermodynamic mutations abrogate drug binding. (b) Simulated effect of mutation on compound off-rates in a model system of a patient over 24 h. Threshold for pharmacological inhibition is 50% of target fraction bound. Coloring scheme consistent with panel A.