Figures & data
Table 1. Mix proportions.
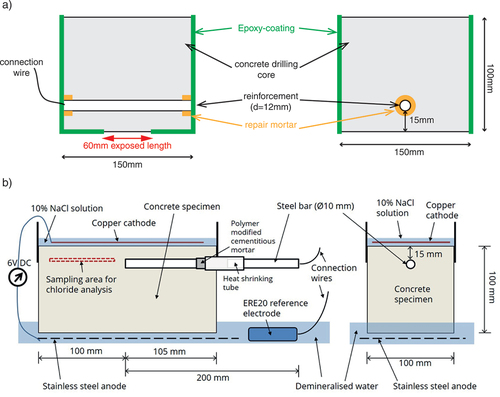
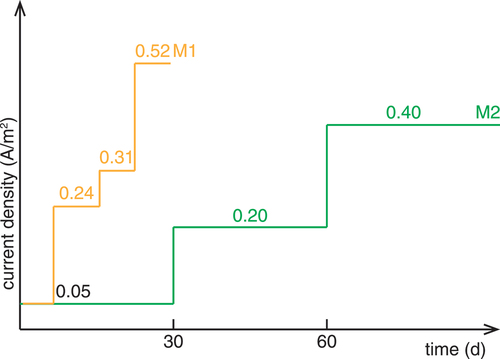
Figure 2. Different exposure methods tested: a) diffusion D b) wetting/drying WD, c) migration 1 M1, d) migration 2 M2, and e) migration M3 and M4. gives an overview of the tested methods for chloride ingress.
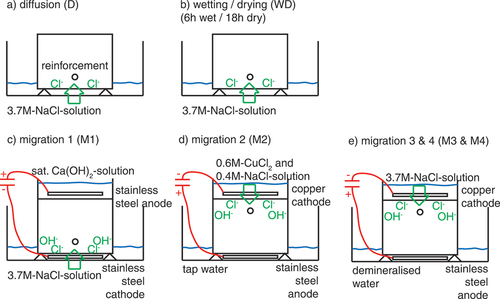
Table 2. Summary of applied techniques for chloride ingress.
Figure 4. Example of measured steel potentials vs. time in experiments at DTI with method M3. Only ‘off’ potentials are shown in the figure, i.e. potentials measured during the periods with applied DC voltage (6 V) has been filtered out. The potential drop observed after about 14 days indicates the onset of corrosion in this particular specimen (‘7_15 mm’). The potentials are measured with an Mn/MnO2 reference electrode but corrected to mV vs. Ag/AgClsat by addition of 222 mV.
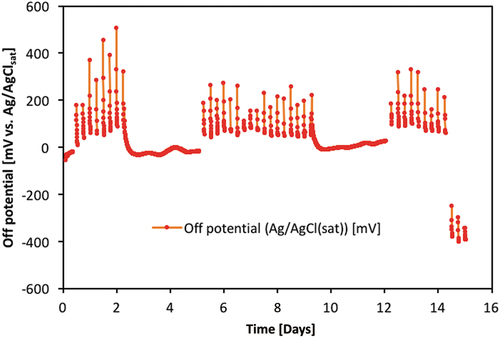
Figure 5. Schematic illustration showing the duration of periods, where the DC voltage (6 V) was only switched off at regular intervals (four times a day) for 1 hour to observe the ‘off’ potential of the steel (continuous, red lines), and periods where the DC voltage was switched off manually (dotted, blue lines). This is shown for both method M3 (a) and M4 (b). A summary of the total time for each DTI specimen, where the DC voltage was turned on and off during the experiments is given in . For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.
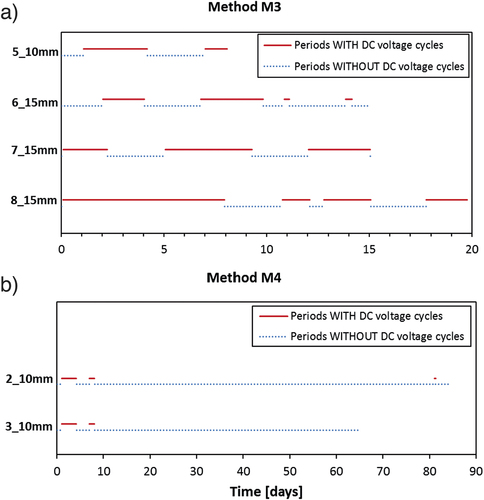
Table 3. Overview of total time with and without applied DC voltage in the experiments with method M3 and M4.
Figure 6. Time to corrosion initiation for the different testing conditions (note that for reasons of comparison, only samples with equal cover depth (15 mm) are shown here). For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.
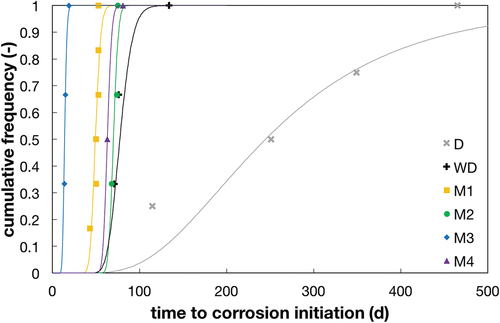
Table 4. Time to corrosion initiation and Ccrit for different accelerating methods. (µ = mean value, = standard deviation).
Figure 7. Box Plots of the Ccrit values for the different methods. The horizontal lines of the boxes represent the 25%, 50%, and 75%-quantiles. The whiskers are the extreme values. Number of specimens per series is D: 4, WD:3, M1: 6, M2: 3, M3: 4 (both cover layers were combined), and M4: 2 (according to ).
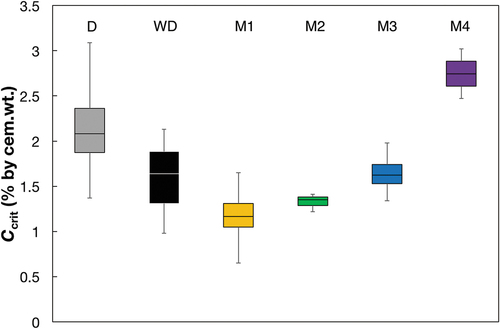
Figure 8. Ccrit vs. time to corrosion initiation, distinguished by acceleration techniques. (note that for reasons of comparison, only samples with equal cover depth (15 mm) are shown here).
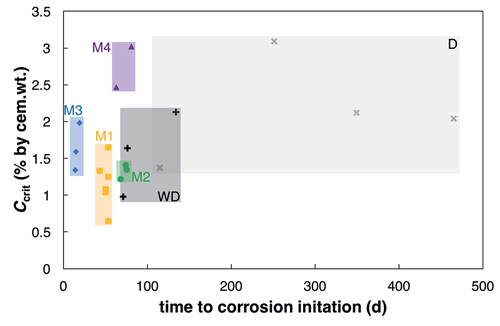
Figure 9. Box plots of potentials before (a) and after (b) initiation of corrosion. The horizontal lines of the boxes represent the 25%, 50%, and 75%-quantiles. The whiskers are the extreme values. Number of specimens per series is D: 4, WD:3, M1: 6, M2: 3, M3: 4 (both cover layers were combined), and M4: 2 (according to ).
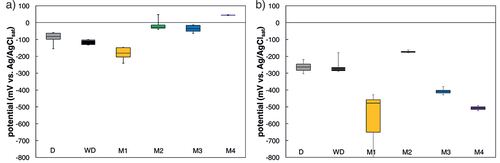
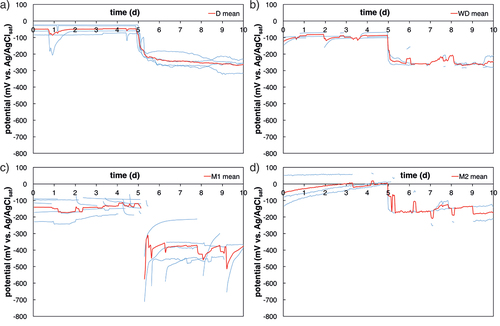
Figure 10. Potential vs. time, 5 days before and 5 days after initiation of corrosion (potential drop > 150 mV). a) is method D, b) method WD, c) method M1, and d) method M2. The red line depicts the mean value, the thin blue lines depict each sample. For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.