Figures & data
Figure 1. Diagrammatic representation of preclinical pain models, tests, and analyses used in genomic studies with relevance to pediatric chronic postsurgical pain phenotypes.
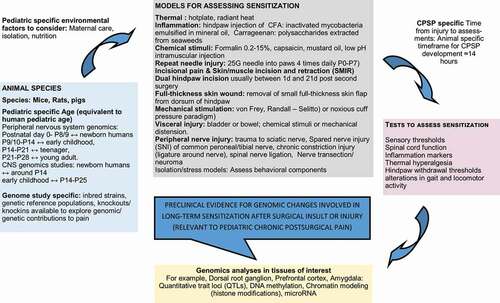
Figure 2. Mechanisms contributing to increased susceptibility to CPSP. Underlying molecular mechanisms comprising genetic variations (i.e., SNPs) and epigenetic modifications (i.e., DNA or histone methylation and acetylation and miRNAs) contribute to individual differences in tissue-specific gene and protein expression in clinical association studies. Gene and protein expression differences can account for increased risk for altered neuronal excitability and sensitization. Alternative mechanisms involved in nociceptive priming are instigated following early life surgery. Tissue injury incites tissue-specific alterations (i.e., epigenetic modifications, gene expression changes) in cell types including sensory neurons and macrophages, which may be important in the formation and maintenance of neonatal nociceptive priming. Underlying conditions and early life surgery can independently contribute to increased susceptibility to CPSP and even act in a feedforward loop together, exacerbating CPSP.
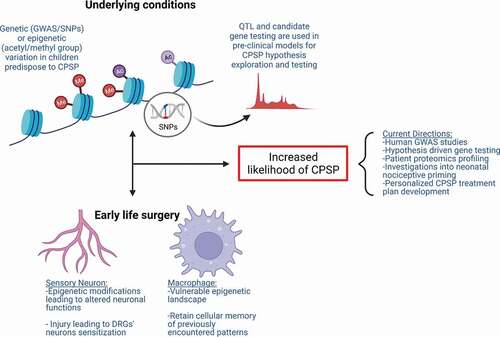
Table 1. Literature-curated list of genes/variants associated with chronic postsurgical pain and their function.
