Figures & data
Table 1. Comparative inhaled corticosteroids (ICS) dosing categories in children, adolescents and adults.
Table 2. Asthma control criteria.Citation5
Figure 1. Approach to suspected uncontrolled severe asthma. *Not meeting the following 8 criteria: daytime symptoms <4 days/week; nighttime symptoms <1 night/week; normal physical activity, mild, infrequent exacerbations; no absence from work or school due to asthma; need for a fast-acting beta2-agonists <4 doses/week, forced expiratory volume in 1 second (FEV1) or peak expiratory flow (PEF) ≥90% of personal best; and PEF diurnal variation <10–15%.Citation1 † Asthma Control Questionnaire on a scale of 0 (totally controlled) to 6 (severely uncontrolled) for individuals aged ≥6 years (in children ≤10 years, it must be administered by a trained interviewer).Citation2,3‡ Asthma Control Test on a scale of 0 (poor control of asthma) to 27 (complete control of asthma) for individuals aged 12 years and older.Citation4,5¶ Child Asthma Control Test on a scale of 0 to 27 for children aged 4 to 11 years old.Citation5,6** Forced expiratory volume in 1 second.†† Forced vital capacity.1Lougheed MD, Lemiere C, Ducharme FM, et al. Canadian Thoracic Society 2012 guideline update: diagnosis and management of asthma in preschoolers, children and adults. Can Respir J 2012; 19(2): 127-64.2Juniper, EF, O'byrne, PM , Guyatt, GH, Ferrie, PJ and King, DR. (1999), Development and validation of a questionnaire to measure asthma control. Eur Respir J, 14: 902–907. doi:10.1034/j.1399-3003.1999.14d29.x3Juniper EF, Svensson K, Mörk AC, Ståhl E. Modification of the Asthma Quality of Life Questionnaire (standardised) in patients 12 years and older. Health and Quality of Life Outcomes 2005, 3:58 ( 16Sep2005 )4Nathan RA, Sorkness CA, Kosinski M, Schatz M, Li JT, Marcus P, Murray JJ, Pendergraft TB. Development of the asthma control test: a survey for assessing asthma control. J Allergy Clin Immunol 2004;113:59-65.5Koolen BB, Pijnenburg MW, Brackel HJ, Landstra AM, Van den Berg NJ, Merkus PJ, Hop WC, Vaessen-Verberne AA. Validation of a web-based version of the asthma control test and childhood asthma control test. Ped Pulmonol 2011;46:941-8.6Liu AH, Zeiger R, Sorkness C, Mahr T, Ostrom N, Burgess S, Rosenzweig JC, Manjunath R. Development and cross-sectional validation of the Childhood Asthma Control Test. J Allergy Clin Immunol 2007;119:817-25.
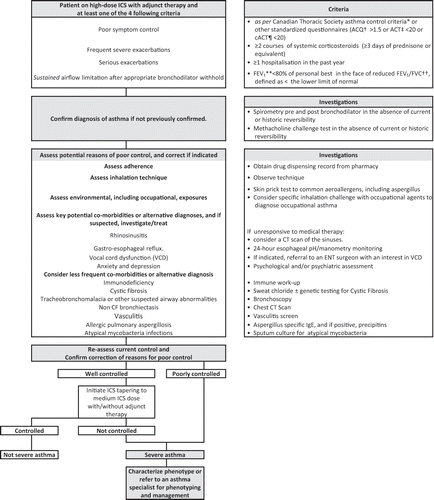
Figure 2. Management hinges upon confirming the diagnosis. All individuals with confirmed asthma should receive self-management education, including a written action plan. Very mild intermittent asthma may be treated with a short-acting beta2 agonist (SABA) taken as needed. SABAs are recommended for relief of symptoms; individuals 12 years of age and over with moderate to severe asthma (particularly those who are exacerbation prone and have poor control) who are taking an ICS/LABA formulation approved also for use as a reliever may do so. Inhaled corticosteroids (ICS) should be introduced early as the initial maintenance treatment for asthma even in individuals who report asthma symptoms less than three times a week. LTRA are second-line monotherapy for mild asthma. If asthma is not adequately controlled by low doses of inhaled corticosteroids, additional therapy should be considered. In children 6 years of age and over, the ICS should be increased to a medium dose before adding an adjunct agent such as a long-acting beta2–agonist (LABA) or LTRA. In individuals 12 years of age and over, a LABA should be considered first as adjunct therapy. A LABA should only be used in combination with an ICS. Increasing to a medium dose of ICS or the addition of a LTRA or tiotropium are third-line therapeutic options. Theophylline may be considered as a fourth-line agent in adults. Severe asthma may require additional treatment as outlined in . Exposure to asthma triggers in the environment, and the presence of co-mordibities should be reassessed at each visit and before altering the maintenance therapy. Consider also assessment of sputum eosinophils in adults with uncontrolled moderate to severe asthma managed in specialialized centres. After achieving acceptable asthma control for at least a few weeks to months, the medication should be reduced to the minimum necessary dose to achieve adequate asthma control and prevent future risk of exacerbations. HFA: Hydrofluoroalkane; mcg: Micrograms; PEF: Peak expiratory flow; yrs: Years.
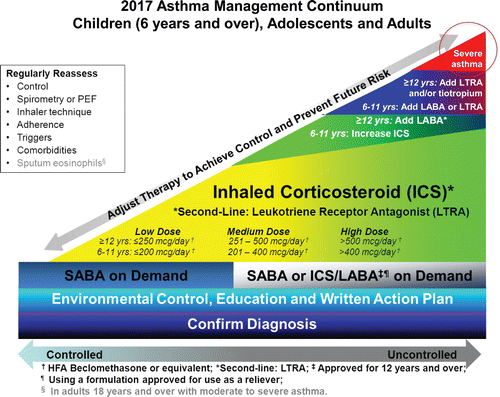
Figure 3. Management of severe asthma: The diagnosis of asthma should be objectively confirmed in all individuals with suspected uncontrolled severe asthma. Domestic and work environment as well as co-morbidities should be thoroughly assessed. Adherence to treatment should also be verified carefully. Individuals with severe asthma should be assessed by an asthma educator in order to ensure adequate inhalation technique and receive a written action plan. Individuals who experience recurrent asthma exacerbations requiring oral corticosteroids in spite of the combination of high dose ICS and long-acting beta 2 agonists +/- leukotriene inhibitor antagonists, theophylline, and/or tiotropium should be phenotyped by measuring blood eosinophil counts and serum IgE +/-sputum eosinophils and/or FeNO. Theophylline has a limited evidence base for use in severe asthma but maybe considered as part of an N-of-One therapeutic trial. Its potential benefits should be balanced against its associated adverse events. Omalizumab may be considered in individuals who experience recurrent asthma exacerbations requiring oral corticosteroids and in prednisone dependent individuals with allergic asthma with positive skin prick test to at least one perennial allergen and total serum IgE between 30 and 1300 IU/mL for children 6-11 years of age and 30 to 700 IU/mL in individuals 12 years of age and over. Anti-IL-5 therapy may be considered in individuals 18 years of age and over who experience recurrent asthma exacerbations requiring oral corticosteroids and prednisone dependent individuals and who show a pre-determined blood eosinophil count cut-off. Macrolides have been shown to decrease asthma exacerbations in only one large RCT and may be considered independently to a specific phenotype. Once asthma control has been achieved consider weaning oral corticosteroids in steroid-dependent individuals and lowering the ICS dose in the other cases.

