Figures & data
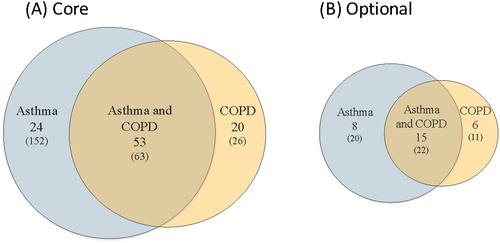
Figure 1. Rating scheme used in Rounds 1 and 2 voting. Ratings were based on 4 domains (strength of evidence, clarity, relevance and feasibility) using a 5-point Likert scale, as well as an overall ranking to include as core, include as optional or do not include for asthma, COPD or both. An example of the results for the category of “Irritant Triggers” is shown.
Figure 2. Flow chart of the initial data element selection process. The Working Group rated 425 potential elements in 4 rounds of voting as core, optional or to be excluded. Elements lacking consensus in a given round were deemed “contentious” and addressed in the next round. Subsequently, duplicate elements were removed. *The sub-totals displayed for asthma and COPD include data elements that are common to both. The element list was further revised based upon data definition review, which included consideration of standardized terminology and reorganization of data element response options into sub-elements.
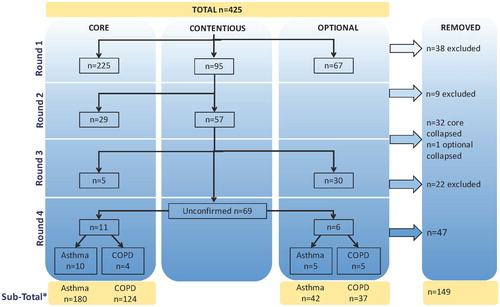
Table 1. Medications by product and brand.