Figures & data
Figure 1. Integrated Comprehensive Management of COPD.Integrated comprehensive management of COPD includes confirming COPD diagnosis with postbronchodilator spirometry, evaluation and on-going monitoring of dyspnea/symptom burden and risk of exacerbations and use of both pharmacologic and nonpharmacologic interventions (see ) to alleviate dyspnea/symptoms, improve health status, prevent AECOPD and reduce mortality. The approach should not be viewed as “stepwise” and may not necessarily occur in the order they appear for all patients. Self-Management Education includes optimizing inhaler device technique and [re-]review, assessment and review of medication adherence, breathing and cough techniques, early recognition of AECOPD, written AECOPD action plan and implementation (when appropriate), promoting physical activity and/or exercise, and other healthy habits including diet and smoking cessation.**Inhaled Maintenance/Preventative Pharmacotherapies are long-acting muscarinic antagonists (LAMA) and/or long-acting ẞ2-agonists (LABA) with or without inhaled corticosteroids (ICS). ICS monotherapy should NOT be used in COPD management.*Other pharmacotherapies include oral therapies (prophylactic macrolide, and PDE-4 inhibitor, mucolytic agents for patients with chronic bronchitis), alpha-1-antitrypsin augmentation therapy for documented severe A1AT deficiency, and opioids for severe refractory dyspnea (see prior CTS Guideline).Citation13ǂSurgical therapies may include lung transplantation and lung volume reduction (including with endoscopic valves).Abbreviations. A1AT, alpha-1 antitrypsin; AECOPD, acute exacerbation of COPD; CAT, COPD assessment test; COPD, chronic obstructive pulmonary disease; CTS, Canadian Thoracic Society; mMRC, modified Medical Research Council; prn, as-needed; NIV, noninvasive ventilation.
![Figure 1. Integrated Comprehensive Management of COPD.Integrated comprehensive management of COPD includes confirming COPD diagnosis with postbronchodilator spirometry, evaluation and on-going monitoring of dyspnea/symptom burden and risk of exacerbations and use of both pharmacologic and nonpharmacologic interventions (see Figure 3) to alleviate dyspnea/symptoms, improve health status, prevent AECOPD and reduce mortality. The approach should not be viewed as “stepwise” and may not necessarily occur in the order they appear for all patients. Self-Management Education includes optimizing inhaler device technique and [re-]review, assessment and review of medication adherence, breathing and cough techniques, early recognition of AECOPD, written AECOPD action plan and implementation (when appropriate), promoting physical activity and/or exercise, and other healthy habits including diet and smoking cessation.**Inhaled Maintenance/Preventative Pharmacotherapies are long-acting muscarinic antagonists (LAMA) and/or long-acting ẞ2-agonists (LABA) with or without inhaled corticosteroids (ICS). ICS monotherapy should NOT be used in COPD management.*Other pharmacotherapies include oral therapies (prophylactic macrolide, and PDE-4 inhibitor, mucolytic agents for patients with chronic bronchitis), alpha-1-antitrypsin augmentation therapy for documented severe A1AT deficiency, and opioids for severe refractory dyspnea (see prior CTS Guideline).Citation13ǂSurgical therapies may include lung transplantation and lung volume reduction (including with endoscopic valves).Abbreviations. A1AT, alpha-1 antitrypsin; AECOPD, acute exacerbation of COPD; CAT, COPD assessment test; COPD, chronic obstructive pulmonary disease; CTS, Canadian Thoracic Society; mMRC, modified Medical Research Council; prn, as-needed; NIV, noninvasive ventilation.](/cms/asset/5127fbc7-82c9-408c-8b89-3306cea8f469/ucts_a_2231451_f0001_c.jpg)
Table 1. 2023 recommendations for PICO 1
How does a clinician choose appropriate maintenance pharmacotherapies in individuals with stable COPD to reduce symptom burden, for example, dyspnea and exercise intolerance, and improve health status?
Table 2. 2023 Recommendations for PICO 2
How does a clinician choose appropriate maintenance pharmacotherapies in individuals with stable COPD to reduce the risk of AECOPD?
Table 3. 2023 Recommendations for PICO 3
How does a clinician choose appropriate maintenance pharmacotherapies in individuals with stable COPD to reduce mortality?
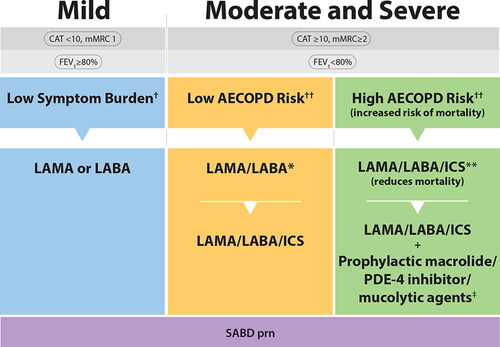
Figure 3. COPD Pharmacotherapy.This figure promotes an evidence-informed approach that aligns proven effective treatments with spirometry, symptom burden, risk of future exacerbations and mortality risk. Because of the clinical heterogeneity in COPD, spirometry should not be used in isolation to assess disease severity and this is why it is also important to perform a thorough clinical evaluation of the patient, including symptom burden and risk of exacerbations that permits the implementation of treatments that are specific for subpopulations. SABD prn (as needed) should accompany all recommended therapies across the spectrum of COPD.†Symptom burden encompasses shortness of breath, activity limitation, and impaired health status.††Individuals are considered at “Low Risk of AECOPD” if ≤1 moderate AECOPD in the last year (moderate AECOPD is an event with prescribed antibiotic and/or oral corticosteroids) and did not require hospital admission/ED visit. Individuals are considered at “High Risk of AECOPD” if ≥2 moderate AECOPD or ≥1 severe exacerbation in the last year (severe AECOPD is an event requiring hospitalization or ED visit).*LAMA/LABA single inhaled dual therapy is preferred over ICS/LABA inhaled combination therapy considering the additional improvements in lung function and the lower rates of adverse events such as pneumonia. ICS/LABA combination therapy should be used in individuals with concomitant asthma. There is no universally accepted definition of concomitant asthma. The 2017 CTS Position Statement on COPD Pharmacotherapy provides guidance on the assessment of patients who may have concomitant asthma.**Triple inhaled ICS/LAMA/LABA combination therapy should preferably be administered in a single inhaler triple therapy (SITT), and not in multiple inhalers (see text), although we acknowledge that some patients continue to prefer separate inhalers. +Oral pharmacotherapies in this group include prophylactic macrolide, and PDE-4 inhibitor and mucolytic agents for patients with chronic bronchitis.Abbreviations. CAT, COPD assessment test; mMRC, Modified Medical Research Council; SABD prn, short-acting bronchodilator as needed; AECOPD, acute exacerbation of COPD; ED, emergency department; LAMA, long-acting muscarinic antagonist; LABA, long-acting ẞ2-agonist; ICS, inhaled corticosteroid.