Figures & data
Table 1. Summary of definitions, frequency, modality for detection, and impact on outcomes of PDL in percutaneous LAAO studies
Figure 2. Examples of current LAAO devices and possible device-specific mechanisms of PDL at the time of device implantation. (a) Single lobe, membrane-coated devices such as the Watchman (Boston Scientific) interact with LAA in one circumferential zone at the level of the LAA landing zone where PDL may occur with these devices. In lobe and disc devices such as the Amplatzer Amulet (Abbott) interact with the LAA at two circumferential territories including the landing zone and then more distally in the body of LAA. These contact sites and the area between the lobe and disc represent potential regions where PDL can occur. The uniformity of LAA-device contact can be influenced by the presence of an additional lobe that can result in off-axis implantation and resultant PDL of both device types. (b) Leak following epicardial LAAO with the LARIAT device is always centrally located and could be modulated by the morphology of the LAA body and ostium. Purple and blue arrows represent (turbulent in some cases) flow into and out of the LAA , respectively. Abbreviations: LA, left atrium; LAA, left atrial appendage; LAAO, LAA occlusion; LSPV, left superior pulmonary vein; LIPV, left inferior pulmonary vein; LLR, left lateral ridge; MV, mitral valve; LV, left ventricle; PDL, peri-device leak; Cx, circumflex artery
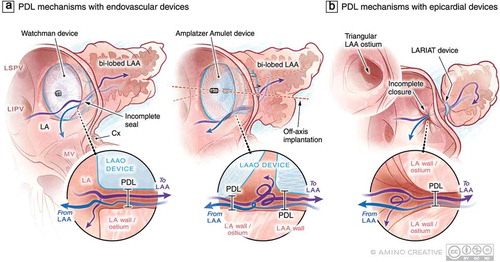
Figure 1. Examples of variability in LAA anatomy that may present challenges to imaging and device sizing and selection. (a) Various shapes of the LAA ostium have been described including round, oval, triangular, and teardrop however the association between LAA ostial shape and successful closure is not well established. (b) Morphologic variation in the body of the LAA are well-recognized including the so-called windsock, cactus, chicken wing, and cauliflower variants as well as the presence of more than one LAA lobe which may impact the success of endovascular device-based closure. Abbreviations: D1, D2 maximum diameters in 2 dimensions; MV, mitral valve; Cx, circumflex artery
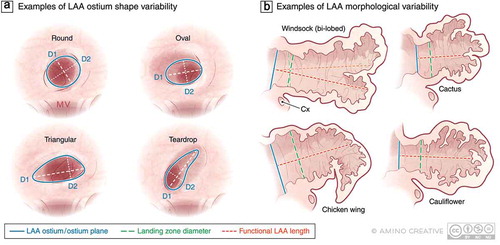
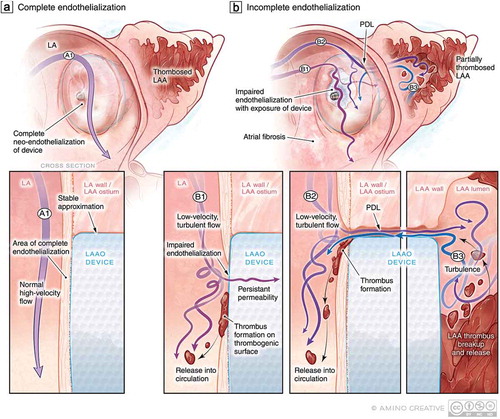
Figure 3. PDL as a precursor to DRT: Putative mechanisms of impaired endothelialization following implantation. (a) Following endovascular LAAO, over time, the LAAO device should ideally be covered by healthy endothelium to prevent thrombogenicity and resultant PDL. (a1) The nature of LA flow (high-velocity laminar vs. low-velocity turbulent) may impact the efficacy of endothelialization. (b) In the context of (b1) low-velocity turbulent flow in the LA, impaired endothelialization may result thereby exposing the thrombogenic surface of the LAAO device to the coagulation system at a time when patients may be discontinuing anticoagulation therapy resulting in DRT and risk of cardioembolism. In the context of PDL (b2), persistent flow in and out of the LAA around the device may also lead to impaired endothelialization and PDT but also delay thrombosis of the LAA and allow for the embolization of LAA thrombi