Figures & data
Table I. Date examined, locality and number of individuals of the Korean greater horseshoe bat, Rhinolophus ferrumequinum korai, tested in this study (all bats were collected from survey sites of abandoned mines).
Figure 1. Photographs showing the size and morphological changes of the testis and epididymis of Korean Rhinolophus ferrumequinum korai according to month. From the middle of October to the middle of March the following year (the hibernating period), the size of testicles gradually decreased. The size of testes began to gradually increase from April (the awakening period). It showed the largest size in August (the active period). A, January; B, February; C, March; D, April; E, May; F, June; G, July; H, August; I, September; J, October; K, November; L, December. Cp, caput epididymis; Cr, corpus epididymis; Cu, cauda epididymis; T, testis.
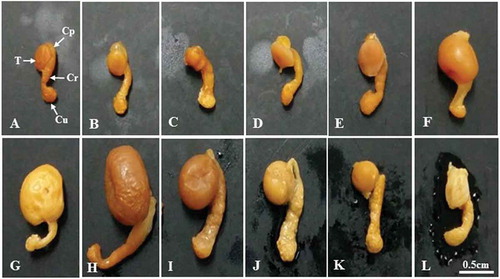
Figure 2. (A–L). Optical microscope photographs showing the stage of differentiation of the seminiferous epithelium over a 1-year cycle. Note that seminiferous tubules from January to March are closed with lumen with only Ad-type spermatogonia and Sertoli cells. Ap-type and In-type spermatogonia as well as primary spermatocytes were observed in the first seminiferous tubules in April. From this period, the lumen began to open. In May, a number of primary spermatocytes were observed in the seminiferous tubules. In June, the earliest sperm cells were observed in the seminiferous tubules. In July, numerous spermatocytes and numerous spermatids were observed in the lumen. In August, there were a few primary spermatocytes, a number of early sperm cells and mature sperm cells, and numerous spermatozoa in the lumen. In September, numerous spermatids were observed in the spermatids and lumen, including many early sperm cells. In October, Ad-type spermatogonia, immature spermatids, and sperm cells were observed. For the first time in this period, many immature spermatids were engulfed as part of the phagocytosis process of Sertoli cells. In November, the pattern of seminiferous tubules was the same as that in October. From this time, the lumen was closed. The lumen of the seminiferous tubules in November and December was also closed. Only Ad-type spermatogonia were observed. Ad, dark type spermatogonium; Ap, pale type spermatogonium; B, B type spermatogonium; Bl, basal lamina; D, diplotene spermatocyte; Es, elongating spermatid; In, intermediate spermatogonium; L, lumen; Lf, lipofuscin; M, meta phase Ms, mature spermatid; P, pachytene spermatocyte; PL-L, pre-leptotene/leptotene spermatocyte; Rs, round spermatid; S, sperm; Se, Sertoli cell; St, spermatid; Z, zygotene spermatogonium. A, January; B, February; C, March; D, April; E, May; F, June; G, July; H, August; I, September; J, October; K, November; L, December.
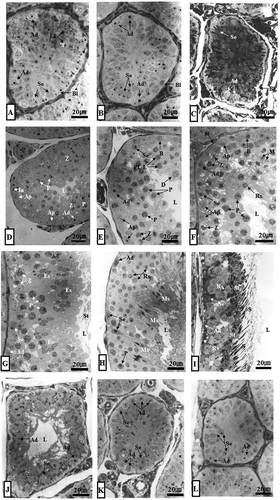
Figure 3. Electron micrographs showing early Golgi phase (June experiment group) and spermiation phase (September experiment group). In the cytoplasm of spherical sperm cells, there were abundant mitochondria and smooth endoplasmic reticulum (sER), including well-developed Golgi complexes (Gc) (A). In addition, many spermatozoa were observed in the lumen, including sperm in the cytoplasm of Sertoli cells (B). L, lumen; N, nucleus; Rb, residual body; S, sperm; Se, Sertoli cell; St, sperm tail.
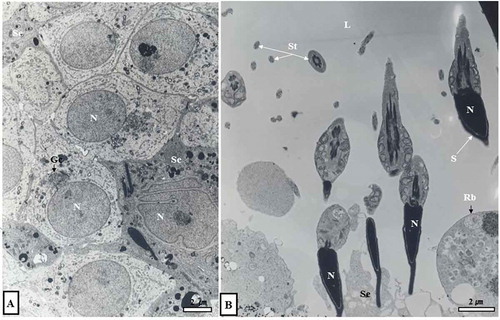
Figure 4. Optical and electron micrographs showing the phagocytosis process of Sertoli cells in seminiferous tubules in October. Note that many immature sperm cells were predated as part of the phagocytosis process of Sertoli cells (A, dotted circle in inset). In the lumen of the seminiferous tubules in October, there were sperm separating from Sertoli cells. Some sperm had already migrated from the lumen to the epididymis (B, inset). In addition, many lipofuscin granules were scattered within the cytoplasm of Sertoli cells (A, B). Ad, dark type spermatogonium; Bl, basal lamina; Irs, immature round spermatid; L, lumen; Lf, lipofuscin; S, sperm; N, nucleus; Se, Sertoli cell.

Figure 5. Optical and electron micrographs showing the phagocytosis process of Sertoli cells in the seminiferous tubules in November. The seminiferous tubules in November, like those in October, were phagocytized to a number of immature spermatids as part of the phagocytosis process of Sertoli cells (A, B). From this point on, the lumen was closed (A, inset, B, inset). Lipofuscin was scattered within the cytoplasm of Sertoli cells (A, B). Ad, dark type spermatogonium; Lf, Lipofuscin; N, nucleus; Pg, phagosome; Se, Sertoli cell; Spt, sperm tail.
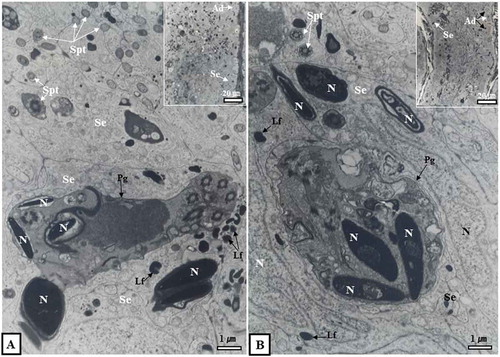
Figure 6. Differentiation stage and periodicity of the seminiferous epithelium according to month. Spermatocytogenesis was in April and May while the process of spermiogenesis was from June to September. The lumen of seminiferous tubules was open from April to October. It was closed from November to March of the following year. Ad, dark-type spermatogonium; Ap, pale-type spermatogonium; B, B-type spermatogonium; D, diplotene spermatocyte; DK, diaknesis; Es, elongating spermatid; In, intermediate spermatogonium; M, metaphase; M/A, metaphase and anaphase of the meiotic cells (meiosis I); Ms, mature spermatid; P, phacytene spermatocyte; PL/L, pre-leptotene/leptotene spermatocyte; Rs, round spermatid; S, sperm; Z, zygotene spermatogonium; □, immature round spermatids.
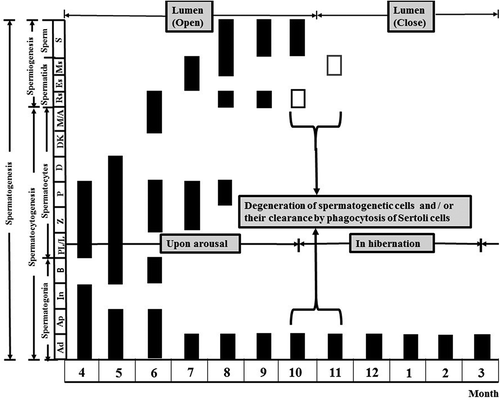
Figure 7. Hibernating Korean Rhinolophus ferrumequinum korai showing three major stages of the male reproductive cycle. The first was the spermatogenesis stage (from April to September), including spermatocytogenesis (from April to May) and the spermiogenesis (from June to early October). The second was the phagocytosis stage (from mid-October to mid-November), which was a purification process to prepare for new spermatogenesis in the following year. The third was a dormant stage. It was a state of holding only spermatogonia and Sertoli cells as an adaptation strategy to make efficient use of energy for long hibernation.
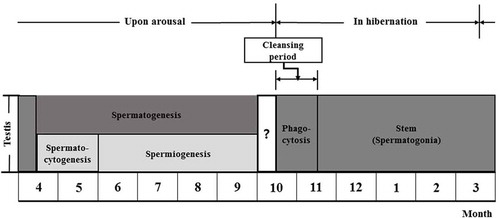
Table II. Comparison of differentiation stages of the seminiferous epithelium in hibernating batspecies in Korea according to month.
Figure 8. Comparison of male reproductive patterns of four kinds of domestic bat species. Note that only immature sperm were killed by Sertoli cells in October and November, just before and immediately after hibernation of Rhinolophus ferrumequinum korai ((b), the present study). Immature spermatozoa shown in Lee et al. (Citation1993) occurred in November (a). In particular, spermatogenesis initiation in this study occurred 1 month earlier than that described in Lee et al. (1993).
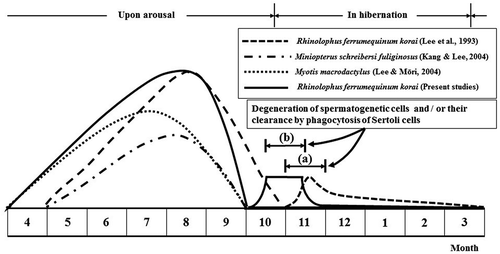
Table III. Comparison of temperature changes from the nearest meteorological stations at the same survey sites between 1991–1992 and 2014–2015 (Korea Meteorological Administration Citation1991, Citation1992, Citation2014, Citation2015).
