Figures & data
Figure 1. (a, b) Type locality of the new species, Central Institute of Temperate Horticulture, Srinagar, Jammu and Kashmir, India.

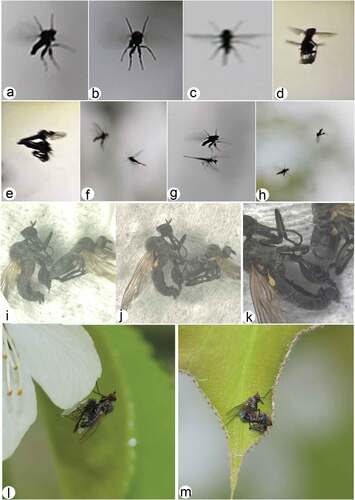
Figure 2. Rhamphomyia aquila sp. nov., male. (a) Head, frontal view; (b) habitus, lateral view; (c) habitus, dorsal view.
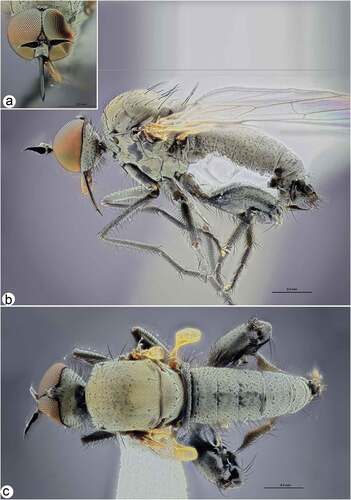
Figure 3. Rhamphomyia aquila sp. nov., male. (a) Mouth parts; (b) antennae; (c–d) legs, male hind leg with deformed femorotibial joint, enlarged view; (e) fore and mid legs; (f) male fore wing with labelled discal cell (dm), cubitus (CuA1) and medial veins (M2); (g–h) male genitalia, cercus (cerc), ejaculatory apodeme (ej apod), epandrium (epand), hypandrium (hypd), phallus (ph).
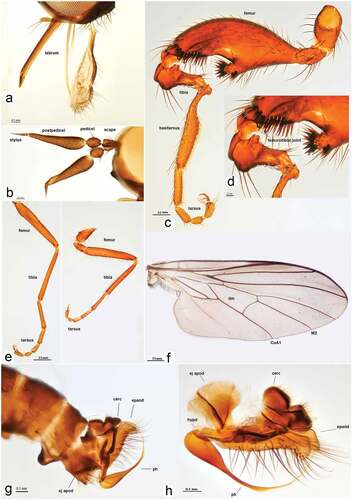
Figure 4. Rhamphomyia aquila sp. nov., female. (a) Head, frontal view; (b) habitus, lateral view; (c) habitus, dorsal view.
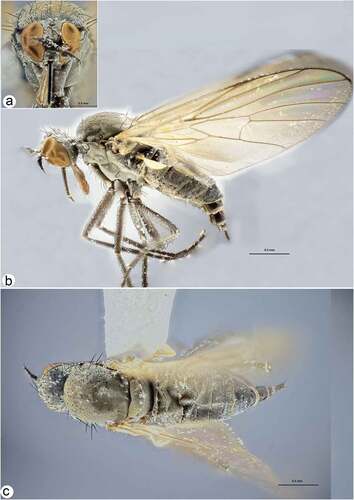
Figure 5. Male and female internal reproductive system of Rhamphomyia aquila sp. nov. (a–d) female internal reproductive tract: (a–b) complete internal reproductive system; (c) right ovary removed and spermatheca extended; (d) separated spermatheca, enlarged view. (e–f) male internal reproductive tract.
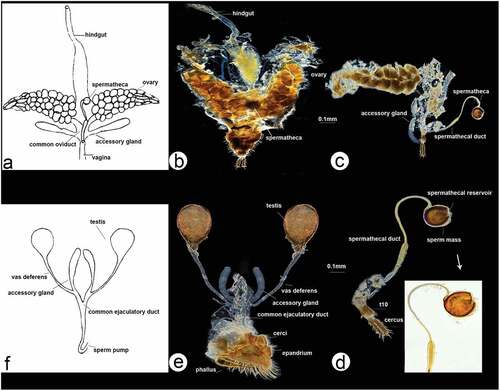
Figure 6. Scanning electron microscopy showing general morphology of (a) female and (b) male of Rhamphomyia aquila sp. nov. body, lateral view; (c) female head, full face view; (d) male head, full face view; (e) female terminalia; (f) male terminalia.
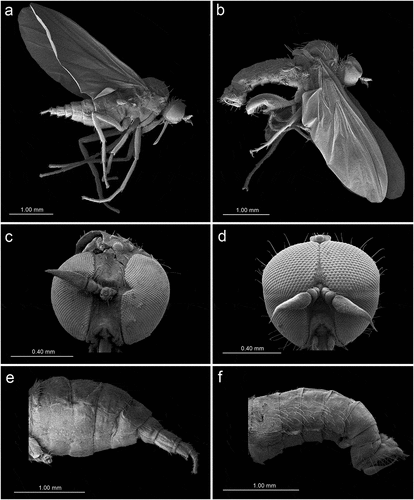
Figure 7. Scanning electron microscopy of Rhamphomyia aquila sp. nov. female head and antennal sensilla. (a) Dichoptic eyes; (b–c) mouth parts with palpi bearing 3–4 long setae (white arrow); (d–e) labium and long stylet labrum with epipharyngeal blades; (f) ventral side of postpedicel with sensilla (white arrows); (g–i) basiconic sensilla (most probably type I) (white star); (j–l) sensilla basiconica with granulated and porous surface.
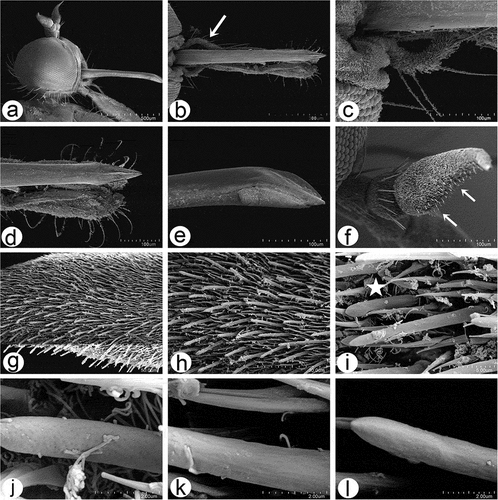
Figure 8. Scanning electron microscopy of Rhamphomyia aquila sp. nov. male head and antennal sensilla. (a–b) Holoptic eyes, with enlarged facets along dorsal half; (c–d) labellum with distinct pseudotracheae; (e) sponge-like general surface of pseudotracheae; (f) labrum with piercing, serrate apical part (white arrows); (g) expanded and convex ventral basal of postpedicel with basiconic sensilla (arrows); (h–k) regularly arranged basiconic sensilla with swollen bases and small pores of sensilla ampullacea (white arrows); (l–o) stylus, mechanoreceptor porous with dense covering of microtrichia.
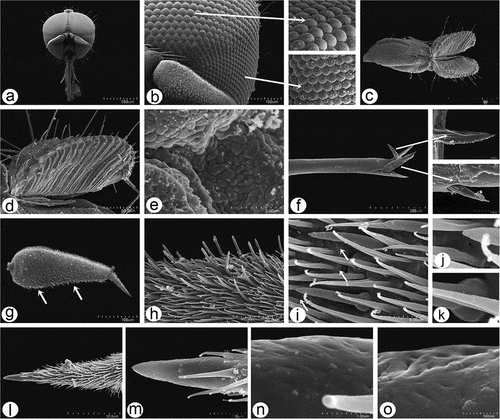
Figure 9. Scanning electron microscopy of Rhamphomyia aquila sp. nov. male antennal sensilla. (a) Basal portion of postpedicel with group of sensilla coeloconica (dotted box); (b) regularly placed sensilla coeloconica (arrows); (c–e) sensilla coeloconica located in depressions (white star); (f–h) peg-shaped sensilla coeloconica surrounded by ring-like cuticular elevation (white dotted arrows).
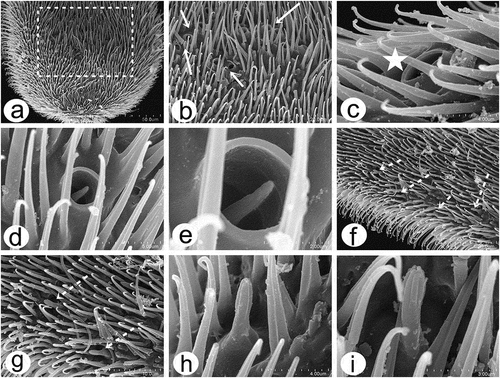
Figure 10. Scanning electron microscopy of Rhamphomyia aquila sp. nov. male detailing wing articulation. (a–c) Microtrichia wing membrane with wax layer; (d–f) long, ribbed chaetic sensilla present along wing edges.
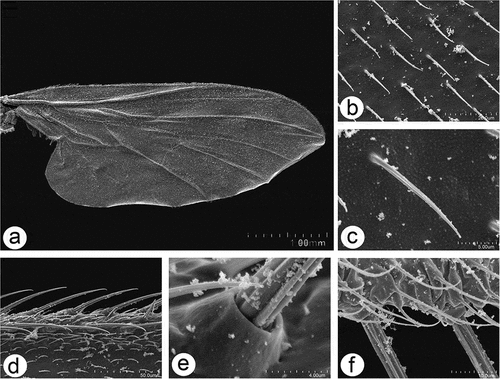
Figure 11. Scanning electron microscopy of Rhamphomyia aquila sp. nov. halter. (a–b) Ventral and dorsal side of the halter with robust scabelum, pedicellus and oval capitellum; (c) sensilla on dorsal scabellum and dorsal pedicellus; (d) scabellus protuberant basal plate sensilla; (e) oriented flanking sensilla on the dorsal pedicel stem; (g–k) single trichoid and campaniform sensillum on capitellum (black arrows : solid, dotted), in addition to the general dense microtrichia.
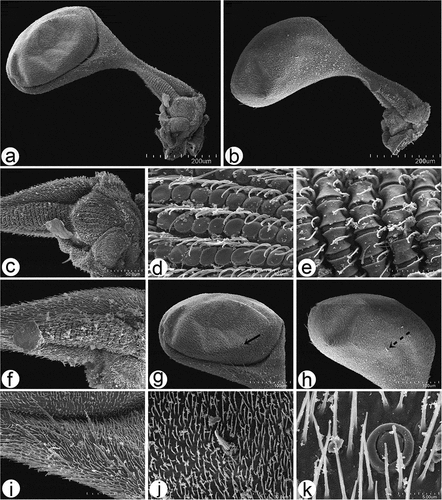
Figure 12. Scanning electron microscopy of Rhamphomyia aquila sp. nov. legs. (a–b) Swollen male hind femur and extremely reduced hind tibia; (c–g) long and stout trichoid and chaetic sensilla on apical third of femur, posteroventrals; (h–j) single campaniform sensillum on the basal inner side of femora (arrow) with small pore near the centre (star); (k–l) elongate basitarsus with large grooved chaetic sensilla; (m–n) trichoid and chaetic sensilla on third tarsomere; (o–q) curved claws and pulvilli (r) fine structure of tenent setae and terminal plates.
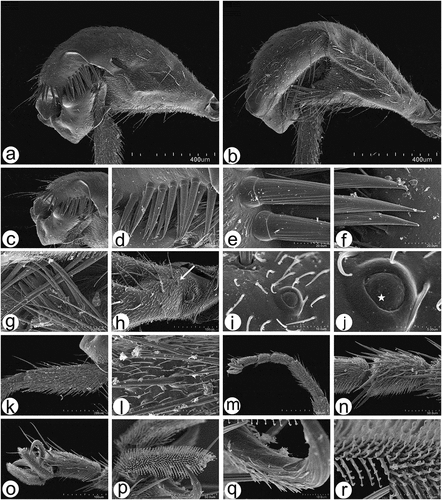
Figure 13. Scanning electron microscopy of Rhamphomyia aquila sp. nov. male and female genitalia. (a) Terminal female abdomen tergites; (b) in ventral view appear partially retracted into proximal segment; (c–e) seventh and eight segments of abdomen with longer chaetic sensilla dorsally; (f) thick, long chaetic sensilla of cerci; (g) smooth basal part of phallus; (h–i) long and rigid chaetic sensilla on genitalia; (j–l) phallus broad, covered by waxy secretions, and bulb-shaped apical portion (arrow).
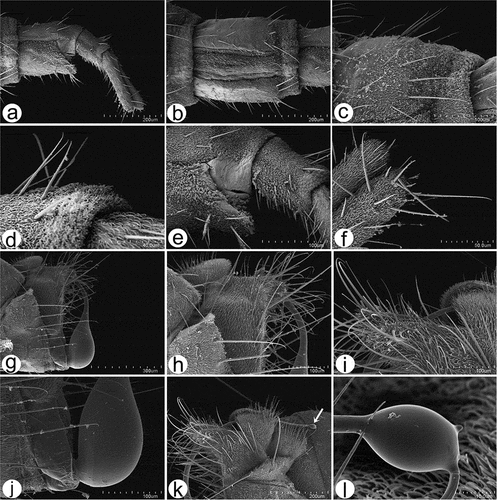
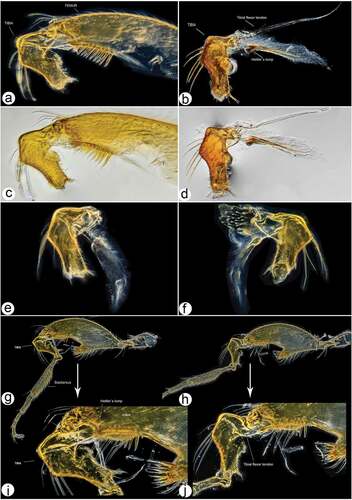
Figure 14. Male hind leg articulation mechanism and involved components. (a–d) Locked femorotibial joint and locking apparatus: (a) darkfield micro image of locked femorotibial joint with labelled tibial flexor sclerite (TFS), genuflexor apodeme (GFS) and Heitler’s lump (HL); (c) bright-field micro image of same structure; (b) bright-field micro image of locking apparatus, lateral view with labelled tibial extensor muscles and Heitler’s lump, and flexor sclerite separated from Heitler’s lump; (d) bright-field micro image of same structure. (e–f) locking apparatus in ventro- and dorso-lateral views; (g–j) femorotibial joint in flexed position (g, i) with tibial flexor sclerite (TFS) and Heitler’s lump in physical contact, and in straight position (h, j) with tibial flexor sclerite (TFS) and Heitler’s lump separated.