Figures & data
Figure 1. Map of all localities in which csotiensis-type eggs were found, including samples collected for the present study (in red). For references, see .
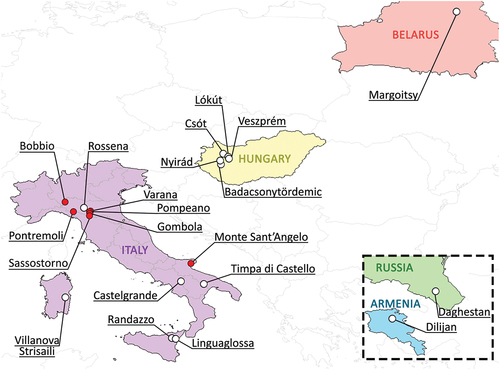
Table I. List of the sampled localities with sample code, coordinates, and altitudes. For each sample, performed analyses are indicated. LM = Light Microscopy; SEM = Scanning Electron Microscopy; CLSM = Confocal Laser Scanning Microscopy.
Table II. Sequence dataset used for molecular analysis of the COI and ITS2 markers, with GenBank accession numbers and codes of the vouchers obtained from the hologenophores or from the cuticles recollected after DNA extraction (a).
Table III. Primer pairs and associated PCR protocols used for the amplification of the ITS2 and COI markers.
Table IV. Summary of all previous records of csotiensis-type egg and its association with areolatus-type egg, with the addition of the samples analyzed in the present study. 1 The two egg morphotypes are reported to be found within the same moss sample; 2 the two egg morphotypes have been certainly found in same locality, but it is not possible to know whether they were also found within the same samples; * egg of Paramacrobiotus klymenki, to be considered of the areolatus type basing on the original description by Pilato et al. (Citation2012), and as also stated in Kaczmarek et al. (Citation2017).
Figure 2. Paramacrobiotus bifrons sp. nov., animal morphology with LM. (a) holotype in toto; (b) eyespots visible in vivo; (c) buccal armature, dorsal view; (d) buccal armature, ventral view; (e) buccal-pharyngeal apparatus; (f) claws of the first pair of legs; (g) claws of the second pair of legs; (h) leg and claw of the third pair, internal side; (i) claws of the fourth pair of legs; (j) fourth pair of legs; (k-l) orcein-stained chromosomes, different planes of the same oocyte; (m) sperms within a sperm duct visible in vivo. Black arrowheads: filament departing from the third macroplacoid; white arrows: cuticular bars under claws; white indented arrows: eyespots; empty arrow: first band of teeth; empty indented arrows: second band of teeth; empty arrowheads: transversal crests; white arrowhead: constriction on the third macroplacoid; white indented arrowheads: granulation on legs; asterisk: pulvinus; empty indented arrowhead: sperms within a sperm duct. a, g–l: PhC; b–f, m: DIC. e–h, j: z-stacks. Scale bars: a = 50 µm; b, e–j, m = 10 µm; c–d = 5 µm; k–l = 2.5 µm.
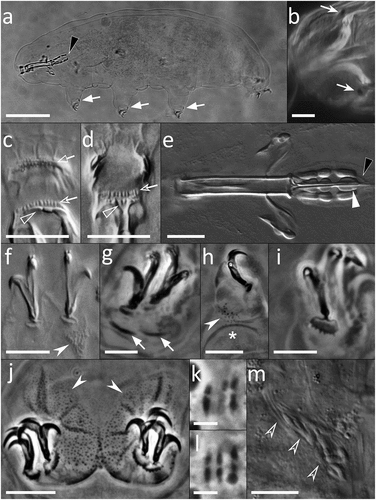
Figure 3. Parsimony haplotype network obtained for the COI gene and parsimony sequence network for the ITS2 fragment of P. areolatus group. The circles are representing different haplotypes and are colored by populations following the legend. The connecting lines are representing single substitutions, while the white dots indicate the missing/ideal haplotypes/sequences. The diameter of the circles is proportional to the number of specimens presenting that specific haplotype/sequence, as indicated in the legend. nwb. = newborn. ASAP partitions for both markers are represented by background-colored boxes: orange background for COI (threshold distance 7.40%; lower ASAP score: 1.00) and grey background for ITS2 (threshold distance 17.89%; lower ASAP score: 1.50). The histograms generated by the ASAP software are showing the frequency distribution of each p-distance in comparison with all the considered P. areolatus group sequences. Red lines are indicating threshold distances.
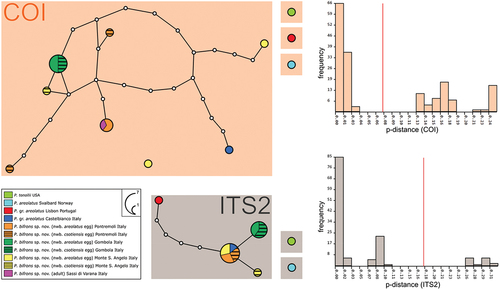
Figure 4. Paramacrobiotus bifrons sp. nov., animal morphology with SEM. (a) in toto; (b) mouth; (c) leg and claws of the first pair, internal side; (d) fourth pair of legs. White arrow: first band of teeth; empty arrowhead: ventral transversal crests; indented arrows: thickening at the base of the claw. Scale bars: a = 100 µm; b–d = 10 µm.
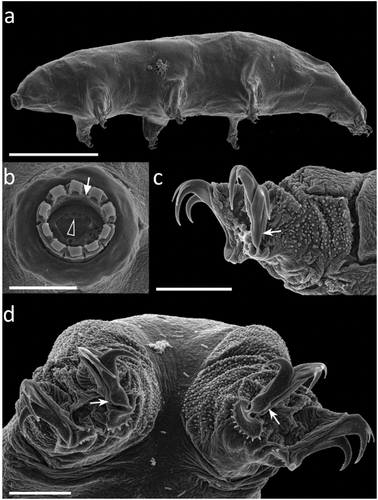
Table V. Paramacrobiotus bifrons sp. nov. List of selected characters measured in animals, including the number of measurements, the range for individual characters expressed in µm and pt, the mean and standard deviation expressed in µm and pt, the measurements of the holotype, and the values obtained from the application of Thorpe’s normalization (parameter b = slope of log Y versus log buccal tube length. Parameter a* = Y-intercept of Thorpe normalized traits versus buccal tube lenght; for the raw data, see supp. Table S4).
Figure 5. Paramacrobiotus bifrons sp. nov., areolatus-type egg with LM. (a) in toto; (b) egg processes; (c) fused egg processes; (d) areolae surrounding a process. White arrow: trabecular layer between the process walls. a–d: PhC, z-stacks. Scale bars: a–c = 10 µm; d = 5 µm.
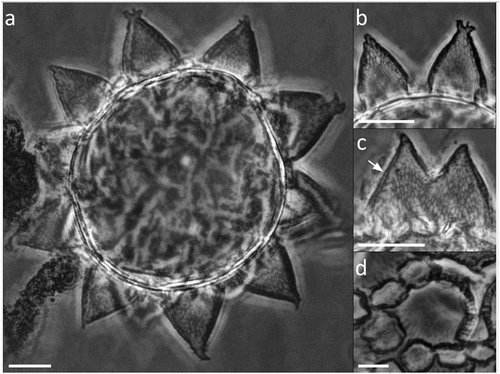
Figure 6. Paramacrobiotus bifrons sp. nov., areolatus-type egg with SEM. (a) in toto, red frame corresponding to (b); (b) basal portion of a process wall with small pores, closeup of (a); (c) two underdeveloped and two normal processes; (d) two fused processes; (e) process surrounded by areolae. White arrow: small pores on the basal portion of a process wall. Scale bars: a,c = 10 µm; b = 2 µm; d–e = 5 µm.
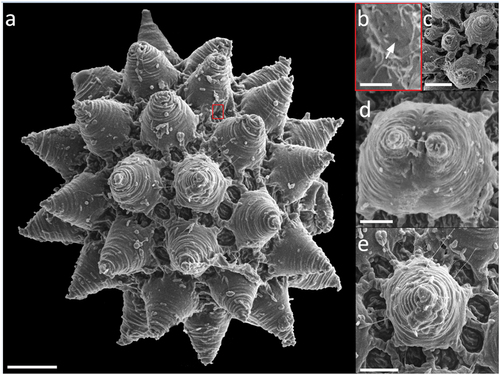
Figure 7. Paramacrobiotus bifrons sp. nov., csotiensis-type egg with LM. (a) in toto; (b) newborn with egg; (c) egg processes in shape of large, fused interconnected crests surrounding a group of areolae; (d) areolae; (e) trabecular layer between the process walls. White arrowheads: crest-shaped egg processes; white indented arrows: groups of areolae; white arrow: trabecular layer between the process walls. a–e: PhC; a–b, d: z-stacks. Scale bars: a,c = 10 µm; b = 25 µm; d = 5 µm; e = 2.5 µm.
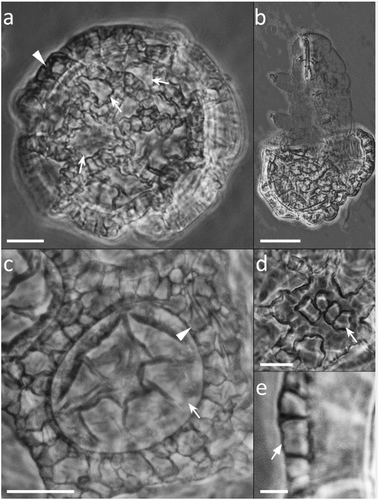
Figure 8. Paramacrobiotus bifrons sp. nov., csotiensis-type egg with SEM. (a–b) in toto; (c–d) basal portion of processes walls with small pores. White indented arrows: areolae; white arrows: small pores on the basal portion of processes walls. Scale bars: a–b = 10 µm; c–d = 2 µm.
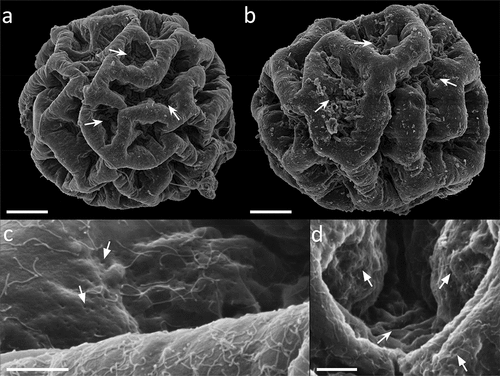
Figure 9. Paramacrobiotus bifrons sp. nov., csotiensis-type eggs aberrations with SEM (a–b) and LM (c–h). (a) in toto egg with small protrusions similar to underdeveloped areolatus-like processes, occurring where three or more crests fuse together; (b) small protrusion similar to areolatus-like process, closeup from (a); (c) in toto egg with aberrant processes appearing as hemispherical; (d) aberrant process appearing as hemispherical, with loose trabecular structures in its upper part, closeup of (c); (e–h) small protrusions similar to areolatus-like processes from various eggs. Arrowhead: csotiensis-type processes in the shape of crests; arrows: small protrusions similar to areolatus-like process; indented arrows: areolae. A-b: SEM; c–h: PhC, z-stacks. Scale bars: a, c = 10 µm; b, d–h = 2.5 µm.
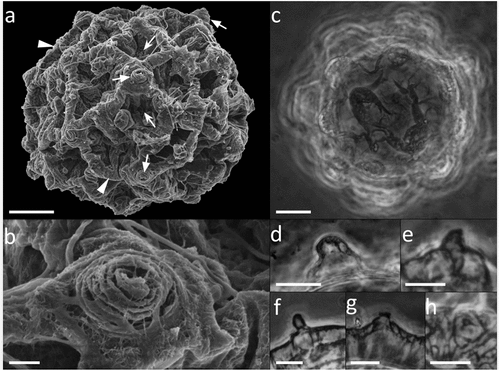
Figure 10. Paramacrobiotus bifrons sp. nov, areolatus- (a–c) and csotiensis- (d–f) type eggs with CLSM, unstained; (a,d) 3D reconstruction of a single hemisphere of the two egg morphotypes, color coded by depth as in the associated scale bar; (b,e) maximum projections of 10 µm sections of (a) and (d), respectively, corresponding to the depth indicated by the white squared bracket on the color-coded-by-depth scale bar; (c,f) cross sections obtained as indicated by the dashed lines in (b) and (e), respectively. b–c, e–f color coded by intensity of autofluorescence signal (from orange, lower, to white, higher). Scale bar: 10 µm.
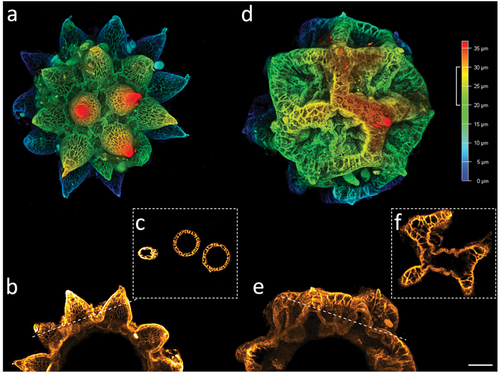
Table VI. Paramacrobiotus bifrons sp. nov. List of selected characters measured in csotiensis- and areolatus- type eggs, including the number of measurements, the range for individual characters, the mean and standard deviation (for the raw data see supp. Table S4).
Table VII. Mean genetic p-distances among the most similar sequences of P. areolatus group present in GenBank. and P. bifrons sp. nov. sequences and mean genetic p-distances inside P. bifrons sp. nov. sequences. Values below the diagonal show p-distances, while values above the diagonal indicate standard deviations.
