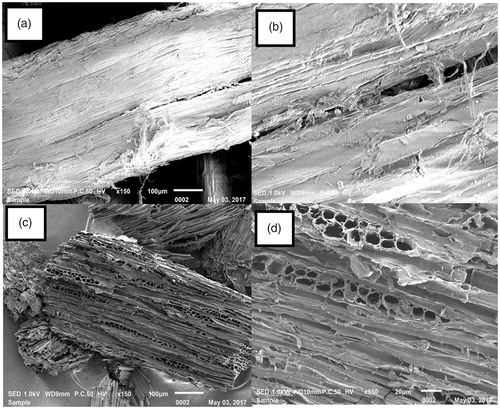
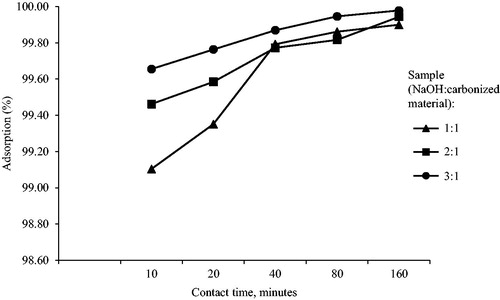
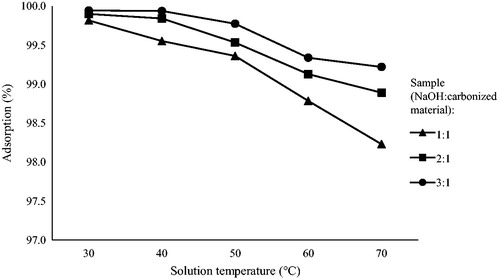
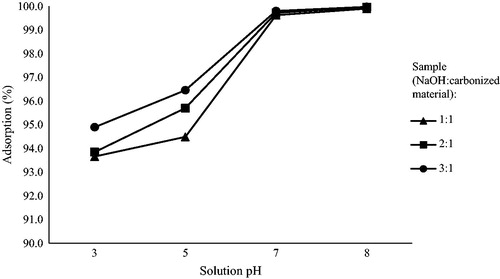
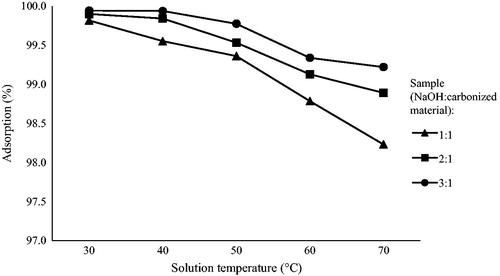
Figures & data
Table 1. Some adsorbents used for removal of cadmium ion from water.
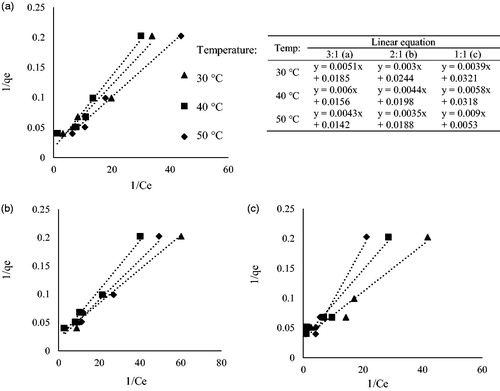
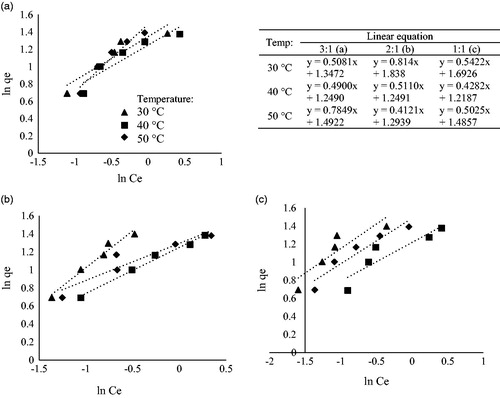
Table 2. Isothermic and kinetics models for the adsorption of Cd2+ by Leucaena leucocephala based activated carbon.
Table 3. Comparison of different adsorption capacity for different cadmium adsorbent.
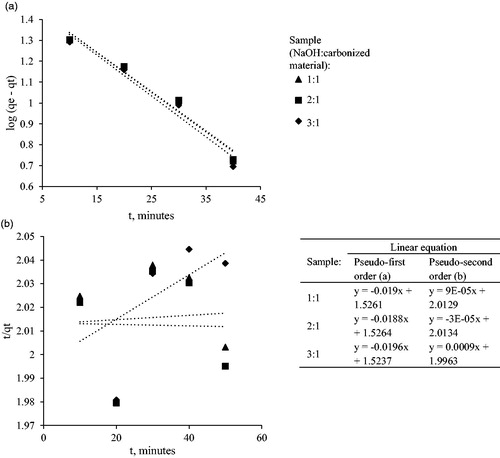
Table 4. Kinetic models for Cd(II) ion adsorption using Leucaena leucephala activated carbon.