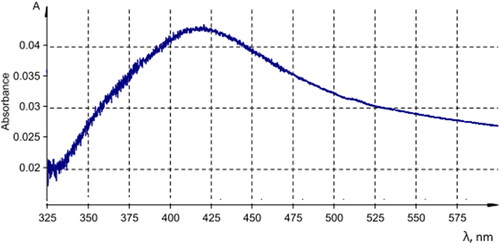
Figures & data
Table 1. Silver concentration for samples of colloidal silver obtained under different reaction conditions.
Figure 3. TEM image for the obtained silver colloids in sample E1(a) (5000× magnification) and sample E2 (b) (16,000× magnification).
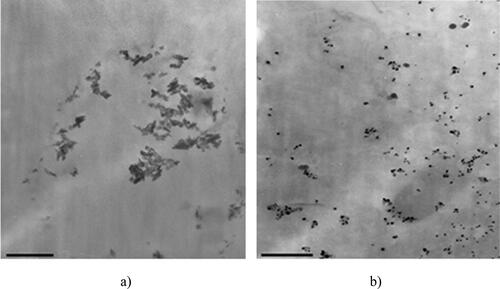
Figure 4. TEM image for the obtained silver colloids in sample E3 (a) and E4 (16,000× magnification).
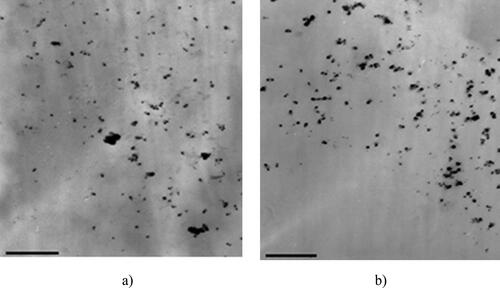
Figure 5. Color changes in the microplate for prepared colloidal silver solutions with different microorganisms.
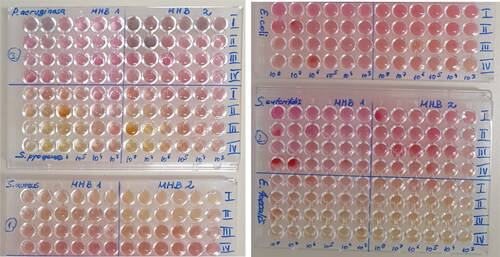
Table 2. Qualitative analysis of the antimicrobial activity of different colloidal silver solutions.