Figures & data
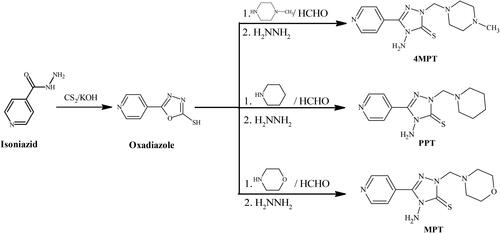
Table 1. Various triazole and Mannich base derivatives were found effective against corrosion.
Figure 2. Nyquist plots for the corrosion control of carbon steel in 1 M HCl in the presence and absence of 4MPT, PPT and MPT.

Figure 3. Bode plots for the corrosion control of carbon steel in 1 M HCl in the presence and absence of 4MPT, PPT and MPT.
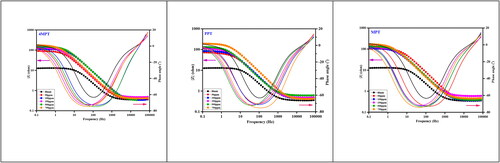
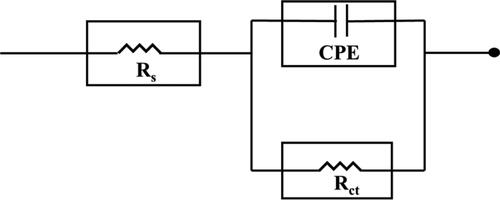
Table 2. The data obtained from fitted EIS curves for corrosion control of carbon steel with the presence and absence of 4MPT, PPT and MPT.
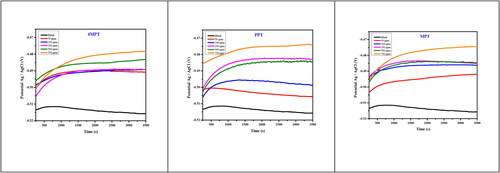
Figure 5. Potentiodynamic polarization plot for the corrosion control of carbon steel in 1 M HCl by different concentration of inhibitors.
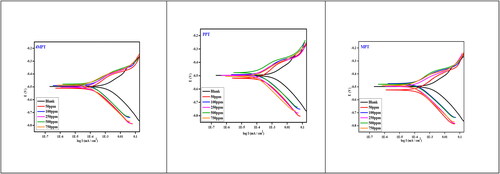
Table 3. Results for the inhibitory effect of 4MPT, PPT and MPT on carbon steel by potentiodynamic polarization and linear polarization resistance experiments.
Table 4. Results for the inhibitory effect of 4MPT, PPT and MPT on carbon steel by electrochemical frequency modulation method.
Table 5. Calculated values of Kads and ΔGoads determined by electrochemical techniques for carbon steel with 4MPT, PPT and MPT.
Figure 6. Scanning electron micrographs (first row) and EDX graphs (second row) of C1018 steel samples: (a) polished fresh metal (b) Immersed in 1 M HCl solution (c–e) Immersed in 1 M HCl solution containing 4MPT, PPT and MPT.
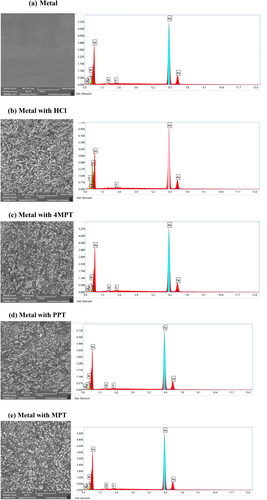
Table 6. The atomic percentage of metal in the presence and absence of 4MPT, PPT and MPT.
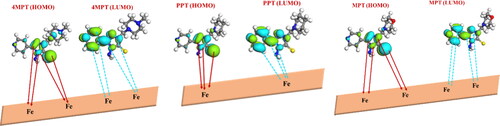
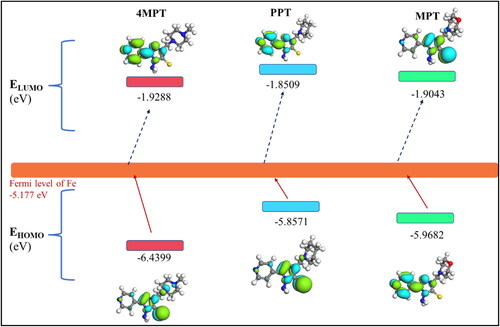
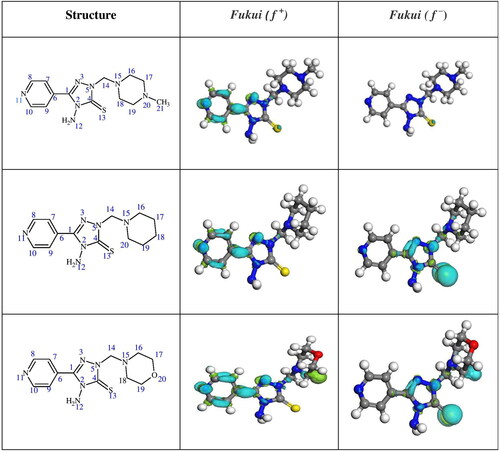
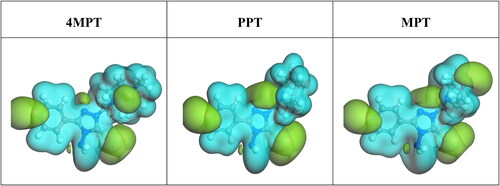
Table 7. Calculated quantum chemical parameters for 4MPT, PPT and MPT by DFT computation study.
Figure 10. Top and side views of the most stable low energy configurations for the adsorption of 4MPT, PPT and MPT molecules on Fe (110)/100 H2O interface obtained using Monte Carlo simulations.
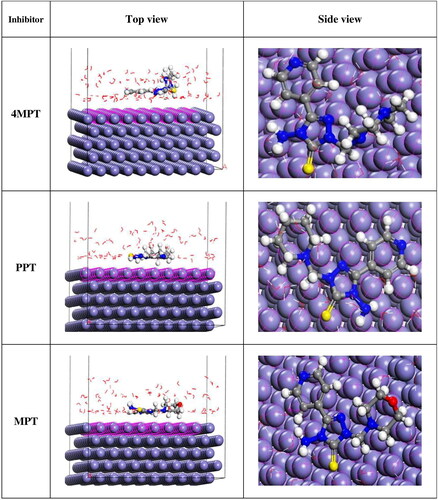
Table 8. The parameters calculated by the Monte Carlo simulation for the most stable adsorption configurations of inhibitor molecules on Fe (110)/100 H2O system (all units in kcal mol−1).
Figure 11. Possible interaction between the surface of C1018 carbon steel and inhibitor 4MPT, PPT and MPT.