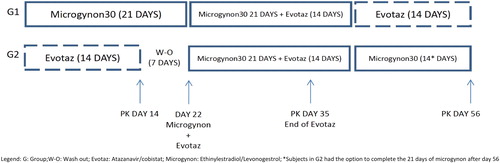
Figures & data
Table 1 Participant demographics, withdrawals and withdrawal reasons
Table 2 Summary of pharmacokinetic data for all four drugs
Figure 2 Levonogestrel (LNG) GM (95% CI) plasma concentration versus time curves, with and without ATV/COBI, GM (95%CI) n = 6.
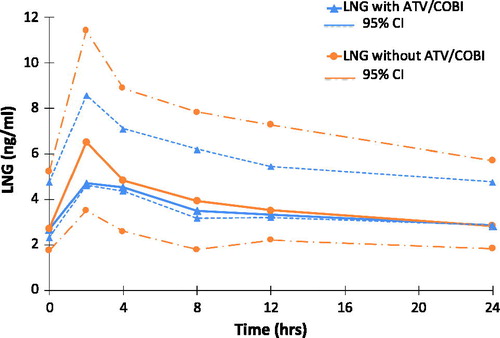
Figure 3 Ethinylestradiol EE GM (95% CI) plasma concentration versus time curves, with and without ATV/COBI, GM (95%CI) n = 6.
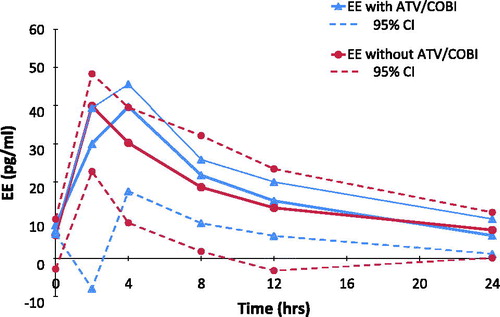
Table 3 Summary of COCP drug interactions studies with atazanavir, ritonavir, and cobicistat